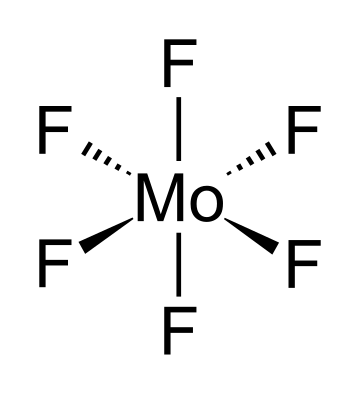
Molybdenum hexafluoride, also molybdenum(VI) fluoride, is the inorganic compound with the formula MoF6. It is the highest fluoride of molybdenum. It is a colourless solid and melts just below room temperature and boils in 34 °C.[3] It is one of the seventeen known binary hexafluorides.
 | |
 | |
| Names | |
|---|---|
| IUPAC names
molybdenum(VI) fluoride | |
| Other names
molybdenum hexafluoride | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.029.114 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| MoF6 | |
| Molar mass | 209.93 g/mol |
| Appearance | white crystals[1] or colorless liquid hygroscopic |
| Density | 3.50 g/cm3[2] |
| Melting point | 17.5 °C (63.5 °F; 290.6 K)[1] |
| Boiling point | 34.0 °C (93.2 °F; 307.1 K)[1] |
| hydrolyzes | |
| −26.0·10−6 cm3/mol | |
| Structure | |
| Orthorhombic, oP28 | |
| Pnma, No. 62 | |
| octahedral (Oh) | |
| 0 | |
| Related compounds | |
Other cations |
Tungsten hexafluoride Uranium hexafluoride Molybdenum(VI) chloride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Synthesis
Molybdenum hexafluoride is made by direct reaction of molybdenum metal in an excess of elemental fluorine:[2]
- Mo + 3 F
2 → MoF
6
The compound hydrolyzes easily,[4] and typical impurities are MoO2F2 and MoOF4.[5]
Description
At −140 °C, it crystallizes in the orthorhombic space group Pnma. Lattice parameters are a = 9.394 Å, b = 8.543 Å, and c = 4.959 Å. There are four formula units (in this case, discrete molecules) per unit cell, giving a density of 3.50 g·cm−3.[2] The fluorine atoms are arranged in the hexagonal close packing.[6]
In liquid and gas phase, MoF6 adopt octahedral molecular geometry with point group Oh. The Mo–F bond length is 1.817 Å.[2]
Applications
Molybdenum hexafluoride has few uses. In the nuclear industry, MoF6 occurs as an impurity in uranium hexafluoride since molybdenum is a fission product of uranium.
The semiconductor industry constructs various integrated circuits through chemical vapor deposition of molybdenum hexafluoride.[4] In some cases, the deposited molybdenum is an impurity in the intended tungsten hexafluoride. MoF6 can be removed by reduction of a WF6-MoF6 mixture with any of a number of elements including hydrogen iodide at moderately elevated temperature.[7][8]
References
Wikiwand in your browser!
Seamless Wikipedia browsing. On steroids.
Every time you click a link to Wikipedia, Wiktionary or Wikiquote in your browser's search results, it will show the modern Wikiwand interface.
Wikiwand extension is a five stars, simple, with minimum permission required to keep your browsing private, safe and transparent.