Top Qs
Timeline
Chat
Perspective
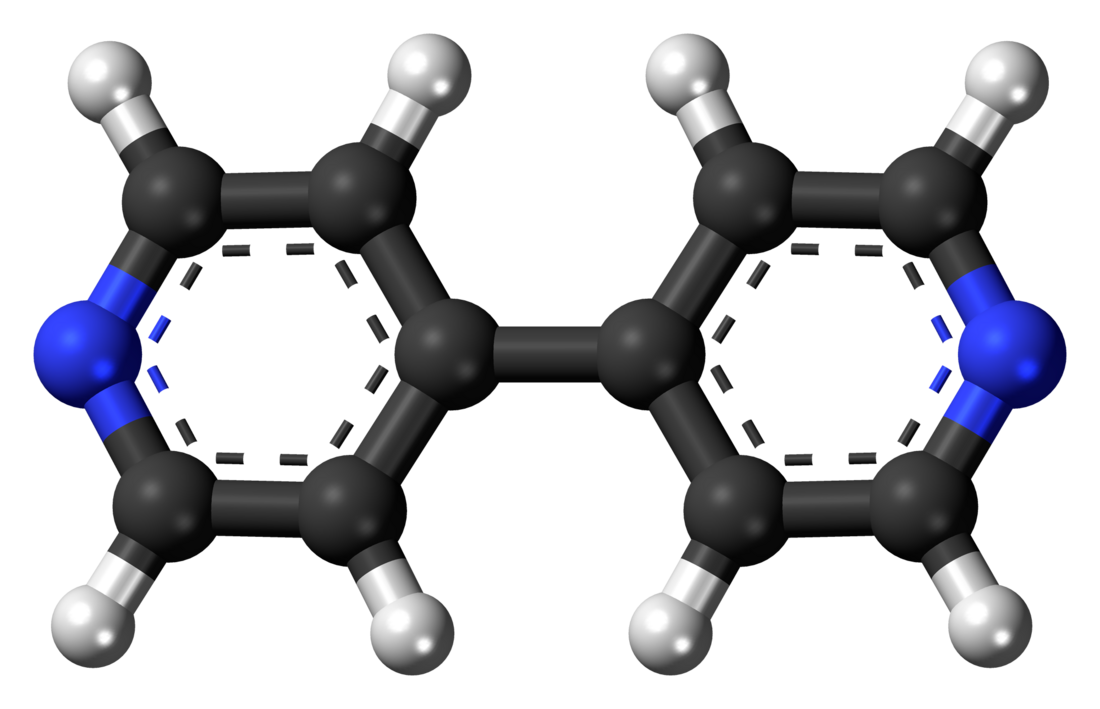
4,4'-Bipyridine
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
4,4′-Bipyridine (abbreviated to 4,4′-bipy or 4,4′-bpy) is an organic compound with the formula (C5H4N)2. It is one of several isomers of bipyridine. It is a colorless solid that is soluble in organic solvents. is mainly used as a precursor to N,N′-dimethyl-4,4′-bipyridinium [(C5H4NCH3)2]2+, known as paraquat.
Remove ads
History
4,4′-Bipyridine was first obtained in 1868 by the Scottish chemist Thomas Anderson via heating pyridine with sodium metal.[1] However, Anderson's empirical formula for 4,4′-bipyridine was incorrect.[2] The correct empirical formula, and the correct molecular structure, for 4,4′-bipyridine was provided in 1882 by the Austrian chemist Hugo Weidel and his student M. Russo.[3]
Uses
4,4'-Bipyridine is an intermediate in the production of paraquat, a widely used herbicide. In this process, pyridine is oxidized to 4,4'-bipyridine in a coupling reaction, followed by dimethylation to form paraquat.[4]
Reactions
The reducing agent N,N'-bis(trimethylsilyl)-4,4'-bipyridinylidene is produced by reduction of 4,4'-bipyridine in the presence of trimethylsilyl chloride (Me = CH3):
- NC5H4C5H4N + 2 Li + 2 Me3SiCl → Me3SiNC5H4C5H4NSiMe3 + 2 LiCl
The silylated derivative, which is red, is used in salt-free reductions.[5]
4,4′-bipyridine forms a variety of coordination polymers.[6]
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads