Top Qs
Timeline
Chat
Perspective
Antineoplastic
Class of drugs used to treat malignant tumors From Wikipedia, the free encyclopedia
Remove ads
Antineoplastic agents, also known as anticancer drugs or antineoplastic drugs, are medications used to treat malignant tumors.[1] These drugs work through various mechanisms to kill or inhibit cancer cells to achieve the goal of treating malignant tumors. Based on their pharmacological actions, antineoplastic drugs can be divided into cytotoxic drugs and non-cytotoxic drugs, with the former primarily consisting of DNA-toxic drugs and the latter mainly comprising molecularly targeted antineoplastic drugs.[2] Commonly used antineoplastic drugs include cisplatin, doxorubicin, paclitaxel, and imatinib.
Traditional cytotoxic drugs, due to their lack of sufficient selectivity for cancer cells, cause varying degrees of damage to normal tissue cells while targeting cancer cells. However, with advancements in tumor molecular biology and translational medicine, antineoplastic drugs have evolved from traditional cytotoxic drugs to non-cytotoxic drugs. Non-cytotoxic drugs are characterized by high selectivity and a high therapeutic index, offering significant clinical advantages.[3]
Remove ads
Uses
Antineoplastic drugs are primarily used in medical settings to treat cancer.[4] Because some antineoplastic drugs also exhibit antiviral activity, they are used to treat certain viral infectious diseases.[5] Certain steroid hormone drugs (used in endocrine therapy), although lacking direct antineoplastic activity, can regulate hormonal balance in the body and suppress certain functional adenocarcinomas, making them commonly used in combination therapies with antineoplastic drugs.[3] Additionally, antineoplastic drugs are employed in scientific research to further understand the molecular biology of cancer through studies of their pharmacological effects.[3]
Remove ads
History
Summarize
Perspective

The first antineoplastic drug, nitrogen mustard, was developed in the 1940s by Louis S. Goodman and Alfred Gilman, Sr. through chemical modification of mustard gas (chemically known as dichlorodiethyl sulfide). Subsequently, chlormethine hydrochloride was approved for clinical use in 1949 as the first antineoplastic drug for treating lymphoma and Hodgkin lymphoma.[1] The first aromatic nitrogen mustard drug, chlorambucil, was approved in 1957 for treating chronic lymphocytic leukemia.[6]
Early antineoplastic drugs were mostly identified through random screening using animal transplantable tumors. Tumor cells exhibit higher phosphoramidase activity than normal cells, and the phosphoryl group, as an electron-withdrawing group, reduces the electron cloud density on the nitrogen atom in nitrogen mustards. Based on this principle, H. Arnold synthesized cyclophosphamide in 1957, which achieved clinical success.[7] In the same year, Charles Heidelberger and colleagues synthesized 5-fluorouracil based on the principle of isoelectronicity, also achieving clinical success.[8] These two drugs were the first effective antineoplastic drugs synthesized based on theoretical principles.[4]
In the early 20th century, Paul Ehrlich proposed the concept of a "magic bullet," envisioning specific compounds that could target drugs to disease sites, reducing damage to normal tissues or cells. This was the initial concept of targeted therapies. In 1948, D. Pressman and G. Keightley suggested using antibodies as cell growth inhibitors and carriers for radionuclides, laying the groundwork for targeted antineoplastic drugs and monoclonal antibody-based therapies.[9] In 1951, W.H. Bellwalt used iodine-131-labeled antibodies to treat thyroid tumors.[10] In 1958, Georges Mathé linked antibodies to methotrexate for treating leukemia. In 1972, T. Ghose and colleagues attached chlorambucil to antibodies to treat melanoma.[11] These experiments validated the feasibility of using antibodies as antineoplastic drugs or carriers, but the antibodies used were polyclonal, with limited specificity and efficacy. In 1975, Georges J. F. Köhler and César Milstein developed monoclonal antibody technology. Due to the high specificity of monoclonal antibodies, targeted antineoplastic drugs began to use them as carriers, leading to the development of numerous monoclonal antibody-based antineoplastic drugs.[12]
Research on the antineoplastic bioactivity of metal platinum complexes began in the 1960s when American physiologist Barnett Rosenberg and colleagues, while studying the effects of electromagnetic fields on microorganism growth, discovered that escherichia coli ceased division and proliferation near platinum electrodes in an ammonium chloride medium. Further studies confirmed that cis-dichlorodiammineplatinum(II) and cis-tetrachlorodiammineplatinum(IV) inhibited cell proliferation. Rosenberg and his collaborators conducted experiments on mice with sarcoma-180 and leukemia L1210, demonstrating cisplatin’s anticancer activity, leading to its entry into clinical trials in 1971.[13][14][15] In 1978, the FDA approved cisplatin for treating testicular cancer and ovarian cancer. The second-generation platinum complex drug carboplatin was introduced in the 1980s, and the first chiral platinum complex drug, oxaliplatin, was approved in 1996.[1]

In 1962, Monroe Eliot Wall and Mansukh C. Wani, began studying the antineoplastic active components of yew tree bark. Wall extracted paclitaxel from the bark of the Pacific yew (Taxus brevifolia) in 1967, with a yield of only 0.014%. Wani used the extracted paclitaxel to prepare single crystals, determining its chemical structure in 1971 through X-ray scattering techniques.[16] In 1979, biologist Susan Band Horwitz identified paclitaxel’s target as tubulin.[17] In 1984, the National Cancer Institute conducted phase I clinical trials of paclitaxel, which showed excellent efficacy against breast cancer and ovarian cancer.[5] In 1989, Robert Anthony Holton of Florida State University extracted paclitaxel’s precursor, 10-deacetylbaccatin (10-DBA), from the leaves of the European yew, with a yield of about 0.1%, and used it for semi-synthetic production of paclitaxel, addressing the issue of insufficient natural paclitaxel yield.[18][19][20][16]
In the late 1990s, Ciba-Geigy (which merged with Sandoz in 1996 to form Novartis)[21] developed the first molecularly targeted antineoplastic drug, imatinib, through targeted screening.[16] In June 1998, imatinib entered phase I clinical trials, and within weeks, the white blood cell counts of the 31 participating patients returned to normal. Just 32 months later, Novartis submitted a new drug application globally, and on March 27, 2001, the FDA granted it priority review status. On May 10, 2001, imatinib was approved for market by the FDA before completing phase III clinical trials, with the approval process being twice as fast as for similar drugs. The successful development of imatinib pioneered a new model for the development of targeted antineoplastic drugs.[22]
Remove ads
Classification
Summarize
Perspective
The variety of antineoplastic drugs used in clinical practice is extensive and rapidly evolving, with classification not yet fully standardized. Generally, they are categorized based on their pharmacological actions and targets.[1][23][24]
General classification
| Cytotoxic drugs | Drugs directly acting on DNA |
|
| Drugs interfering with DNA Synthesis (Antimetabolites) |
| |
| Drugs acting on structural proteins |
| |
| Non-Cytotoxic Drugs | Molecularly targeted drugs |
|
| Other antineoplastic drugs |
|
Specific drug types
Remove ads
Mechanism of action
Summarize
Perspective

Tumor cell populations include proliferating cells, quiescent cells (G0 phase), and non-proliferative cells. The ratio of proliferating tumor cells to the total tumor cell population is called the growth fraction (GF). The time from the end of one cell division to the end of the next is called the cell cycle, which consists of four phases: pre-DNA synthesis (G1 phase), DNA synthesis (S phase), post-DNA synthesis (G2 phase), and mitosis (M phase).[4]
Cytotoxic drugs
Cytotoxic drugs exert cytotoxic effects on tumor cells in different phases of the cell cycle and delay phase transitions by affecting biochemical events.[25] Based on their sensitivity to tumor cells in specific phases, cytotoxic drugs are broadly divided into two categories:
- Cell cycle non-specific agents (CCNSA): These drugs kill cells in various phases of the proliferative cycle, including G0 phase cells, such as drugs that directly damage DNA structure or affect its replication or transcription functions (e.g., alkylating agents, antitumor antibiotics, and platinum complexes). These drugs often have a strong effect on malignant tumor cells, rapidly killing them in a dose-dependent manner, with effects increasing exponentially within the body’s tolerable toxicity limits.[23]
- Cell cycle (phase) specific agents (CCSA): These drugs are sensitive only to specific phases of the proliferative cycle and not to G0 phase cells, such as antimetabolites acting on S-phase cells and vinblastine drugs acting on M-phase cells. These drugs have a weaker effect on tumor cells, with time-dependent cytotoxicity, requiring a certain duration to take effect, and their efficacy does not increase beyond a certain dose.[23]

Non-cytotoxic drugs
Non-cytotoxic drugs primarily target key regulatory molecules in tumor molecular pathology processes.[3] Examples include hormones or their antagonists that alter hormone imbalance; protein tyrosine kinase inhibitors, farnesyltransferase inhibitors, MAPK signaling pathway inhibitors, and cell cycle regulators targeting cell signal transduction molecules; monoclonal antibodies targeting proliferation-related cell signal transduction receptors; angiogenesis inhibitors that disrupt or inhibit new blood vessel formation, effectively preventing tumor growth and metastasis; anti-metastatic drugs that reduce cancer cell shedding, adhesion, and basement membrane degradation; and inhibitors targeting telomerase to promote differentiation of malignant tumor cells.[23]
Remove ads
Toxicology
Summarize
Perspective
Currently, clinically used cytotoxic drugs lack ideal selectivity for tumor cells versus normal cells, meaning that while killing malignant tumor cells, they also cause some degree of damage to normal tissues. Toxic reactions are a key factor limiting the dosage used in chemotherapy and also affect patients’ quality of life.[27] Some molecularly targeted drugs in non-cytotoxic drugs, such as tumor signaling pathway inhibitors, can specifically target certain molecular sites in tumor cells that are typically not expressed or minimally expressed in normal cells. Therefore, non-cytotoxic drugs generally have high safety, good tolerability, and milder toxic reactions.[28]
Adverse reactions of cytotoxic drugs

Common toxic reactions
- Bone marrow suppression: One of the major obstacles in cancer chemotherapy, most cytotoxic drugs, except hormones, bleomycin, and L-asparaginase, cause varying degrees of bone marrow suppression. The likelihood of reduced peripheral blood cell counts after chemotherapy depends on cell lifespan, with shorter-lived peripheral blood cells more likely to decrease, typically starting with leukopenia followed by thrombocytopenia, generally without causing severe anemia. In addition to using colony-stimulating factors such as GM-CSF, G-CSF, M-CSF, and EPO to manage blood cell decline, nursing care must include measures to prevent infections and control bleeding.[28]
- Gastrointestinal reactions: The most common toxic reaction of cytotoxic drugs. Chemotherapy-induced nausea and vomiting are classified into acute (within 24 hours of chemotherapy) and delayed (after 24 hours). For high or moderate emetogenic drugs, dexamethasone and 5-HT3 receptor antagonists (e.g., ondansetron) may be used, while mild emetogenic drugs can be managed with metoclopramide or chlorpromazine. Chemotherapy can also damage rapidly proliferating gastrointestinal mucosal tissues, easily causing stomatitis, oral ulcers, glossitis, and esophagitis, necessitating attention to oral hygiene to prevent infections.[28]
- Hair loss: Normal human scalp has about 100,000 hairs, with 10%–15% of hair-generating cells in the resting phase, while the majority are actively growing, making most cytotoxic drugs capable of causing varying degrees of hair loss. During chemotherapy, using an ice cap to cool the scalp, causing local vasoconstriction, or applying a tourniquet at the hairline can reduce drug delivery to hair follicles, mitigating hair loss. Hair can regrow after chemotherapy cessation.[29]
Specific toxic reactions

- Cardiac toxicity: Most common with doxorubicin, which can cause myocardial degeneration and interstitial edema. Cardiac toxicity may be related to doxorubicin-induced free radical generation.[28]
- Respiratory system toxicity: Primarily manifests as interstitial pneumonia and pulmonary fibrosis, with key drugs including bleomycin, carmustine, mitomycin C, methotrexate, and gefitinib. Long-term high-dose bleomycin use can cause interstitial pneumonia and pulmonary fibrosis, possibly due to the lack of bleomycin-inactivating enzymes in lung endothelial cells.[30]
- Liver toxicity: Some cytotoxic drugs, such as L-asparaginase, dactinomycin, and cyclophosphamide, can cause liver damage.[30]
- Urinary system toxicity: High-dose cyclophosphamide can cause hemorrhagic cystitis, possibly due to large amounts of the metabolite acrolein excreted through the urinary tract; co-administration of mesna can prevent this. Cisplatin, secreted by renal tubules, can damage proximal and distal tubules. Maintaining adequate urine output can help reduce urinary system toxicity.[29]
- Neurotoxicity: Vincristine is most likely to cause peripheral neuropathy. Cisplatin, methotrexate, and 5-fluorouracil may occasionally cause some neurotoxicity.[29]
- Hypersensitivity reaction: Antineoplastic drugs that are polypeptides or proteins, such as L-asparaginase and bleomycin, are likely to cause hypersensitivity reactions when administered intravenously. Paclitaxel’s hypersensitivity reactions may be related to the excipient polyoxyethylated castor oil.[29]
- Tissue necrosis and deep vein thrombosis: Highly irritating drugs, such as mitomycin C and doxorubicin, can cause thrombophlebitis at the injection site, and extravasation of the injection solution can lead to local tissue necrosis, necessitating proper injection techniques.[27]
Long-term toxic reactions
- Second primary malignant tumors: Many antineoplastic drugs, particularly alkylating agents, are mutagenic and carcinogenic, and have immunosuppressive effects. In patients who achieve long-term survival after chemotherapy, some may develop second primary malignant tumors potentially related to chemotherapy.[27]
- Infertility and teratogenicity: Many antineoplastic drugs, especially alkylating agents, can affect germ cell production and endocrine function, causing infertility and teratogenic effects. In male patients, testicular germ cell numbers significantly decrease, leading to male infertility; in female patients, permanent ovarian dysfunction and amenorrhea may occur, and in pregnant women, miscarriage or teratogenesis may result.[30]
Adverse reactions of non-cytotoxic drugs
Non-cytotoxic drugs have milder toxic reactions but still exhibit some side effects.[30]
Monoclonal antibody drugs
Monoclonal antibody drugs are classified into murine monoclonal antibodies, chimeric monoclonal antibodies, humanized monoclonal antibodies, and fully humanized monoclonal antibodies. Murine monoclonal antibodies (drugs with “-momab” as the generic name suffix) have good specificity and rapid metabolism but, due to their lack of humanized components, induce human anti-mouse antibodies, resulting in significant side effects.[31] Due to these significant side effects, no new murine monoclonal antibody drugs have entered clinical research since 2003.[22] Chimeric monoclonal antibodies (drugs with “-ximab” as the generic name suffix) are composed of the variable (V) region of murine monoclonal antibodies spliced with the constant (C) region of human antibodies, with human components accounting for 60%–70%, reducing side effects while retaining antigen-binding specificity.[31] Humanized monoclonal antibodies (drugs with “-zumab” or “-umab” as the generic name suffix) replace the CDR of human antibodies with that of murine monoclonal antibodies, with human components accounting for about 90%, further reducing side effects but slightly decreasing antigen-binding capacity.[31] Fully humanized monoclonal antibodies (drugs with “-mumab” or “-umab” as the generic name suffix) are produced by gene knockout technology, replacing mouse antibody genes with human antibody genes, followed by immunization with antigens and hybridoma techniques. With 100% human components, they have minimal side effects and unaffected therapeutic efficacy.[31]
Small-molecule kinase inhibitors
Due to their high specificity, small-molecule kinase inhibitors have minimal side effects, with gastrointestinal reactions being the most common.[28] Inhibitors targeting epidermal growth factor receptor (EGFR) and vascular endothelial growth factor receptor (VEGFR), such as gefitinib, can affect the patient’s circulatory system, leading to hypertension and high blood sugar side effects.[1]
Remove ads
Drug resistance
Summarize
Perspective
Tumor cells developing resistance to antineoplastic drugs is a major cause of chemotherapy failure.[2] Some tumor cells exhibit natural resistance, where they are inherently insensitive to certain drugs, such as G0 phase tumor cells, which are generally insensitive to most antineoplastic drugs. Other tumor cells develop acquired resistance, becoming insensitive to drugs they were initially sensitive to after a period of treatment.[32] The most prominent and common form of resistance is multiple drug resistance (MDR) or pleiotropic drug resistance, where tumor cells develop resistance to multiple structurally and mechanistically diverse antineoplastic drugs after exposure to one drug.[27] The mechanisms of drug resistance are complex, varying by drug and involving multiple resistance mechanisms for the same drug. The genetic basis of resistance has been established, with tumor cells having a fixed mutation rate during proliferation, each mutation potentially leading to resistant tumor strains. Thus, the larger the tumor (i.e., the more divisions), the greater the chance of resistant strains emerging. The tumor stem cell hypothesis suggests that tumor stem cells are a primary cause of chemotherapy failure, with drug resistance being one of their characteristics.[4] Modern research indicates that tumor cells are more likely to develop resistance to molecularly targeted drugs.[27]
Remove ads
Pharmaceutics
Summarize
Perspective
Due to the lack of selectivity of cytotoxic drugs, they cause significant side effects.[2] In addition to developing new non-cytotoxic drugs to reduce side effects, modifying the dosage forms of cytotoxic drugs is an important strategy. In 1906, Paul Ehrlich proposed the concept of targeted drug systems. Targeted formulations, considered the fourth generation of drug dosage forms, are deemed suitable for antineoplastic drugs.[34] These formulations enhance the specificity of non-cytotoxic drugs and confer selectivity to cytotoxic drugs.
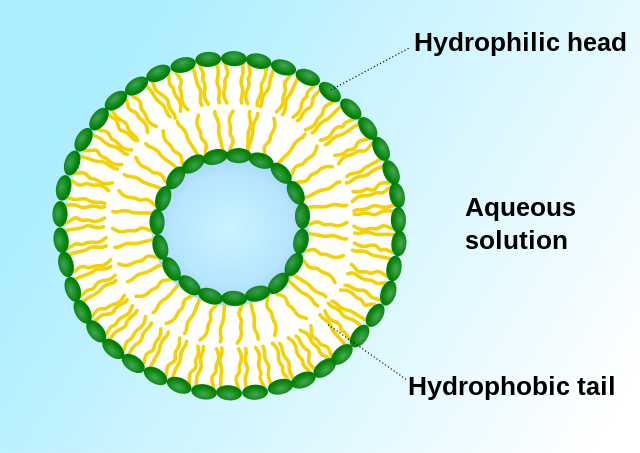
Early targeted formulations were primarily passive.[12] In 1961, British hematologist Alec Bangham invented liposomes.[35][36][37] In 1971, liposomes were first used as drug carriers, marking the earliest passive targeted formulation.[38] Liposomes enable drugs to selectively kill or inhibit cancer cell proliferation, increasing selectivity for lymphoid tissues. Since tumor cells contain higher concentrations of phosphatases and acylases than normal cells, encapsulating anticancer drugs in liposomes facilitates drug release due to enzymatic action and enhances drug retention in target areas.[39][40] Active targeted formulations include modified drug carriers (e.g., ibuprofen zinc microemulsion), prodrugs (e.g., cyclophosphamide), and drug-macromolecule complexes. Due to their higher selectivity, active targeted formulations deliver drugs directly to the target area, enhancing therapeutic efficacy.[12]
With advances in molecular biology, research on physicochemical targeted formulations has deepened. These include magnetic targeted formulations, embolism targeted formulations, thermosensitive targeted formulations, and pH-sensitive targeted formulations. Magnetic targeted formulations encapsulate drugs with ferromagnetic materials in polymeric carriers, guided by external magnetic fields for targeted delivery and localization in the body, primarily used as anticancer drug carriers. Embolism targeted formulations block blood supply and nutrients to the target area, causing ischemic necrosis of cancer cells. Embolism formulations containing antineoplastic drugs combine embolization with targeted chemotherapy. pH-sensitive formulations exploit the significantly lower pH of tumor interstitial fluid compared to surrounding normal tissues for targeted therapy.[12]
Remove ads
Preparation methods
Most antineoplastic drugs are industrially prepared through total or semi-synthesis, while a few drugs (e.g., polypeptide or protein-based antineoplastic drugs) are produced on a large scale through biopharmaceutical methods or natural component extraction.[1]
Future development
Summarize
Perspective

With a deeper understanding of tumor pathogenesis and the regulation of cell differentiation, proliferation, and apoptosis at the molecular level, antineoplastic drugs have shifted from traditional cytotoxic effects to targeting multiple molecular pathways.[16] Newly marketed molecular targeted antineoplastic drugs can be divided into small molecule chemical drugs and biotechnology drugs. The former primarily consist of various small molecule kinase inhibitors, alongside proteasome inhibitors and some epigenetic drugs. The latter, represented by monoclonal antibody drugs, are increasingly becoming a cornerstone of cancer therapy. These drugs surpass traditional direct cytotoxic agents.[6] The development of molecular targeted antineoplastic drugs is currently a hot topic in drug development.[15]
Target-based drug development
Current methods for developing targets for antineoplastic drugs include: identifying targets from effective monomeric compounds; discovering targets based on differences in gene expression between normal and pathological tissues; identifying targets through quantitative analysis and comparative studies of changes in protein expression profiles in normal versus diseased states; discovering targets based on protein interactions; and using RNA interference technology to specifically suppress the expression of different genes in cells, identifying targets through changes in cellular phenotype. The development of new antineoplastic drugs involves using the three-dimensional structure of targets, employing computer-aided drug design to rapidly screen for lead compounds, and subsequently obtaining the target drug. The primary targets for new targeted antineoplastic drugs are divided into genomics and proteomics. Currently, targeted antineoplastic drugs focus on two main types of driver genes: one is receptor molecules located on the cell membrane (e.g., HER2/neu), and the other is molecules in key intracellular signaling pathways (e.g., EGFR). Mutations such as insertions, deletions, rearrangements, or amplifications activate driver genes, conferring adaptability to cancer cells, thus driving cancer development and progression. Protein targets for targeted antineoplastic drugs mainly include disease-specific proteins (e.g., polypeptide Op18, heat shock protein 70), biomarker molecules (e.g., cellular keratin CK19), and enzyme molecules (e.g., histone deacetylase (HDAC)).
Remove ads
See also
Notes
- Strictly speaking, these drugs belong to endocrine therapy drugs (ATC code L02), but due to their widespread use in cancer treatment, they are listed here.
References
External links
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads