Top Qs
Timeline
Chat
Perspective
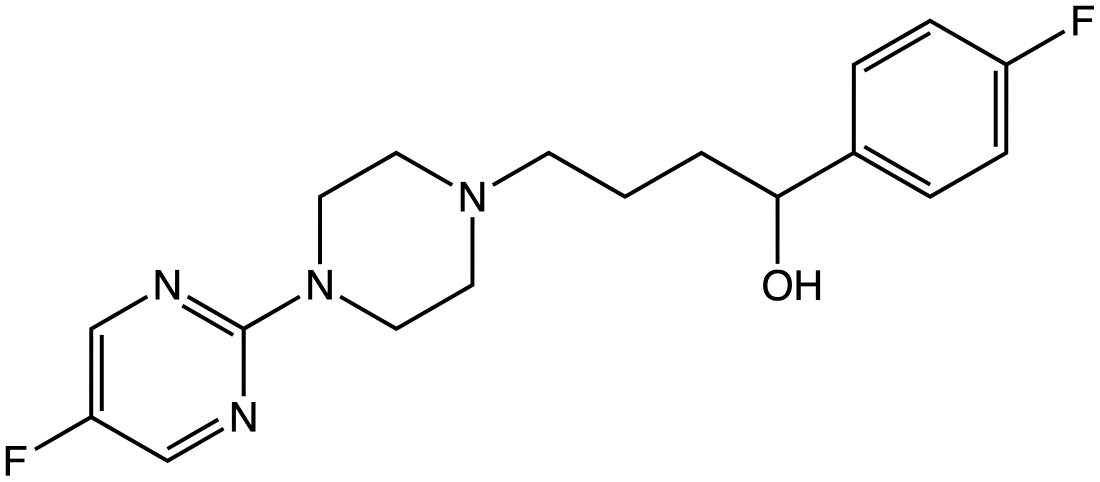
BMY-14802
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
BMY-14802, also known as BMS-181100, is a drug with antipsychotic effects which acts as both a sigma receptor antagonist and a 5-HT1A receptor agonist.[1][2][3][4] It also has affinity for the 5-HT2 and D4 receptors.[5] The drug reached phase III clinical trials for the treatment of psychosis but was never marketed.[6]
Remove ads
Synthesis
Patent (Ex1/2/5/6/7):[7]

The reaction of 4-chloro-4'-fluorobutyrophenone [3874-54-2] (1) with ethylene glycol gives the ketal, 2-(3-chloropropyl)-2-(4-fluorophenyl)-1,3-dioxolane [3308-94-9] (2). The reaction of 2-chloro-5-fluoro-4-methylthiopyrimidine [87789-51-3] (3) with N-carboethoxypiperazine [120-43-4] (4) gives ethyl-4-(5-fluoro-4-methylthio-2-pyrimidinyl)-1-piperazine carboxylate, PC10470079 (5). Catalytic hydrogenation removes the thiomethyl group giving ethyl-4-(5-fluoro-2-pyrimidinyl)-1-piperazine carboxylate [87789-52-4] (6). Acid hydrolysis of the carbamate protecting group gives a secondary amine and hence 5-fluoro-2-(piperazin-1-yl)pyrimidine [87789-49-9] (7). Alkylation of 2 with 7 and subsequent hydrolysis of the ketal protecting group afforded 1-(4-fluorophenyl)-4-(4-(5-fluoro-2-pyrimidinyl)-1-piperazinyl)butan-1-one [133982-66-8] (8). Lastly, reduction of the benzoyl ketone with sodium borohydride gave the alcohol, completing the synthesis of BMY-14802 (9).
Remove ads
See also
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads