Top Qs
Timeline
Chat
Perspective
Chloroalkyl ether
From Wikipedia, the free encyclopedia
Remove ads
Chloroalkyl ethers are a class of organic compounds with the general structure R-O-(CH2)n-Cl, characterized as an ether connected to a chloromethyl group via an alkane chain.
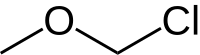
Chloromethyl methyl ether (CMME) is an ether with the formula CH3OCH2Cl. It is used as an alkylating agent and industrial solvent to manufacture dodecylbenzyl chloride, water repellents, ion-exchange resins, polymers, and as a chloromethylation reagent. In organic synthesis the compound is used for the introduction of the methoxymethyl (MOM) protecting group.
Closely related compounds of industrial importance are bis(chloromethyl) ether (BCME) (closely related to chemical weapon sulfur mustard)[1] and benzyl chloromethyl ether (BOMCl).
| Chloromethyl ether | R | Chemical structure | Molar mass (g/mol) | CAS number | Boiling point (°C) |
| Benzyl chloromethyl ether | Benzyl | 156.61 | 3587-60-8 | 102 °C @ 14 mmHg (1.9 kPa) | |
| Chloromethyl methyl ether | Methyl | 80.51 | 107-30-2 | 55-57 | |
| Bis(chloromethyl) ether | Chloromethyl | 114.96 | 542-88-1 | 106 | |
| tert-Butyl chloromethyl ether | tert-Butyl | 124.5 | 40556-01-2 | ||
| 2-Methoxyethoxymethyl chloride | Methoxyethyl | 124.57 | 3970-21-6 | 50-52 °C @ 13 mmHg (1.7 kPa) | |
| Dichloromethyl methyl ether | Methyl |  | 114.96 | 4885-02-3 | 82 - 85.5 °C |
| Representative chloroalkyl ethers[2] | |||||
Methoxymethyl ethers (MOMs) and methoxyethyl ethers (MEMs) are common protecting groups in organic synthesis.
Remove ads
Safety
Chloroalkyl ethers are strong alkylating agents with attendant dangers. These compounds are human carcinogen.[3]
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads
