Top Qs
Timeline
Chat
Perspective
Carbon–nitrogen bond
Covalent bond From Wikipedia, the free encyclopedia
Remove ads
A carbon–nitrogen bond is a covalent bond between carbon and nitrogen and is one of the most abundant bonds in organic chemistry and biochemistry.[1]
Nitrogen has five valence electrons and in simple amines it is trivalent, with the two remaining electrons forming a lone pair. Through that pair, nitrogen can form an additional bond to hydrogen making it tetravalent and with a positive charge in ammonium salts. Many nitrogen compounds can thus be potentially basic but its degree depends on the configuration: the nitrogen atom in amides is not basic due to delocalization of the lone pair into a double bond and in pyrrole the lone pair is part of an aromatic sextet.
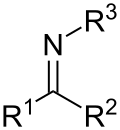
Similar to carbon–carbon bonds, these bonds can form stable double bonds, as in imines; and triple bonds, such as nitriles. Bond lengths range from 147.9 pm for simple amines to 147.5 pm for C-N= compounds such as nitromethane to 135.2 pm for partial double bonds in pyridine to 115.8 pm for triple bonds as in nitriles.[2]
A CN bond is strongly polarized towards nitrogen (the electronegativities of C and N are 2.55 and 3.04, respectively) and subsequently molecular dipole moments can be high: cyanamide 4.27 D, diazomethane 1.5 D, methyl azide 2.17, pyridine 2.19. For this reason many compounds containing CN bonds are water-soluble. N-philes are group of radical molecules which are specifically attracted to the C=N bonds.[3]
Carbon-nitrogen bond can be analyzed by X-ray photoelectron spectroscopy (XPS). Depending on the bonding states the peak positions differ in N1s XPS spectra.[4][5][6]
Remove ads
Nitrogen functional groups
Remove ads
See also
- Cyanide
- Other carbon bonds with group 15 elements: carbon–nitrogen bonds, carbon–phosphorus bonds
- Other carbon bonds with period 2 elements: carbon–lithium bonds, carbon–beryllium bonds, carbon–boron bonds, carbon–carbon bonds, carbon–nitrogen bonds, carbon–oxygen bonds, carbon–fluorine bonds
- Carbon–hydrogen bond
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads