Top Qs
Timeline
Chat
Perspective
Thiophosgene
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
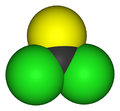
Thiophosgene is a red liquid with the formula CSCl2. It is a molecule with trigonal planar geometry. There are two reactive C–Cl bonds that allow it to be used in diverse organic syntheses.[1]
Remove ads
Preparation
Typically, CSCl2 is prepared in a two-step process from carbon disulfide. In the first step, carbon disulfide is chlorinated to give trichloromethanesulfenyl chloride (CCl3SCl), a rare sulfenyl chloride:
- CS2 + 3 Cl2 → CCl3SCl + S2Cl2
The chlorination must be controlled as excess chlorine converts trichloromethanesulfenyl chloride into carbon tetrachloride. Steam distillation separates the trichloromethanesulfenyl chloride and hydrolyzes the disulfur dichloride. Reduction of trichloromethanesulfenyl chloride with, e.g., tin[2] or dihydroanthracene[3] produces thiophosgene:
- CCl3SCl + M → CSCl2 + MCl2
An alternative one-step reaction is[4]
- CCl4 + H2S → SCCl2 + 2 HCl
Remove ads
Reactions
CSCl2 is mainly used to prepare compounds with the connectivity CSX2 where X = OR, NHR.[5] Such reactions proceed via intermediate such as CSClX. Under certain conditions, one can convert primary amines into isothiocyanates. CSCl2 also serves as a dienophile to give, after reduction 5-thiacyclohexene derivatives. Thiophosgene is also known as the appropriate reagent in Corey-Winter synthesis for stereospecific conversion of 1,2-diols into alkenes.[6]
It forms a head-to-tail dimer upon irradiation with UV light:
- 2 CSCl2 → S2(CCl2)2
Unlike thiophosgene monomer, a red liquid, the photodimer, an example of a 1,3-dithietane, is a colourless solid.[7] Swarts fluorination of the dimer and then cracking is the principal route to thiocarbonyl fluoride.[8]
Thiophosgene decomposes at 200 °C or above to form carbon disulfide and carbon tetrachloride.[9] It has also been observed decomposing to hydrogen sulfide, hydrogen chloride, and carbonyl sulfide gases via contact with human tissue.[10][failed verification]
Remove ads
Toxicology and safety
CSCl2 is considered highly toxic. Inhalation of the substance can cause irritation of respiratory system, burns, delayed pulmonary edema and death.[11]
See also
References
Further reading
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads