Top Qs
Timeline
Chat
Perspective
Dichlorine hexoxide
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
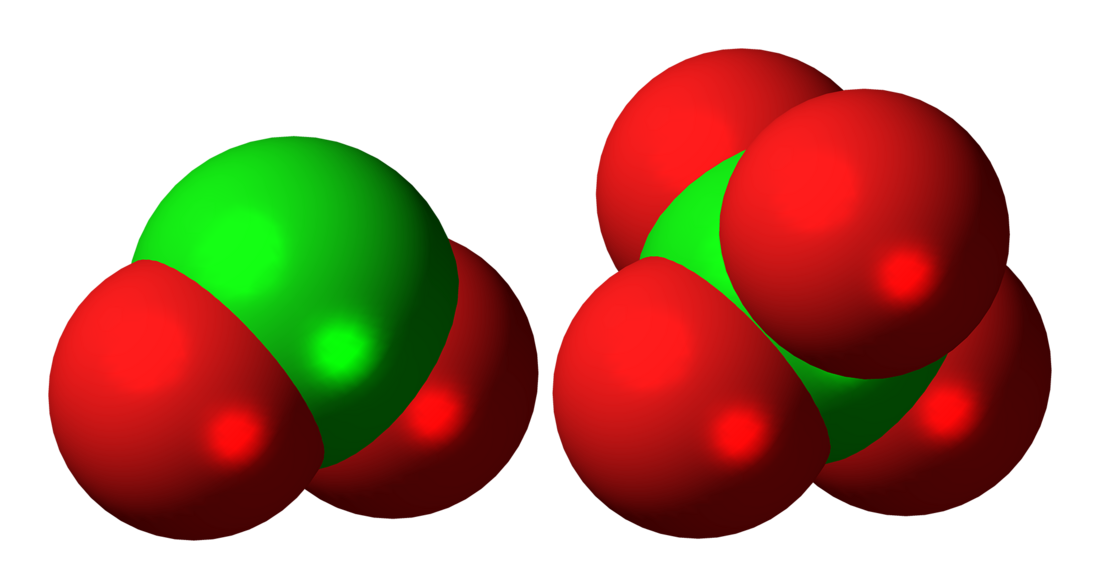
Dichlorine hexoxide is the chemical compound with the molecular formula Cl2O6 or O2Cl−O−ClO3, which is correct for its gaseous state. However, in liquid or solid form, this chlorine oxide ionizes into the dark red ionic compound chloryl perchlorate or dioxochloronium(V) perchlorate [ClO2]+[ClO4]−, which may be thought of as the mixed anhydride of chloric and perchloric acids. This compound is a notable perchlorating agent.[1]
Remove ads
Molecular structure
It was originally reported to exist as the monomeric chlorine trioxide ClO3 in gas phase,[2] but was later shown to remain an oxygen-bridged dimer after evaporation and until thermal decomposition into chlorine perchlorate, Cl2O4, and oxygen.[3] The compound ClO3 was then rediscovered.[4]
It is a dark red fuming liquid at room temperature that crystallizes as a red ionic compound, chloryl perchlorate, [ClO2]+[ClO4]−. The red color shows the presence of chloryl ions. Thus, chlorine's formal oxidation state in this compound remains a mixture of chlorine(V) and chlorine(VII) both in the gas phase and when condensed; however by breaking one oxygen-chlorine bond some electron density does shifts towards the chlorine(VII).
Remove ads
Properties
Cl2O6 is diamagnetic and is a very strong oxidizing agent. Although stable at room temperature, it explodes violently on contact with organic compounds[5] It is a strong dehydrating agent:
Many reactions involving Cl2O6 reflect its ionic structure, [ClO2]+[ClO4]−, including the following:[6]
- NO2F + Cl2O6 → [NO2]+ClO−4 + ClO2F
- NO + Cl2O6 → [NO]+ClO−4 + ClO2
- 2 V2O5 + 12 Cl2O6 → 4 VO(ClO4)3 + 12 ClO2 + 3 O2
- SnCl4 + 6 Cl2O6 → [ClO2]2[Sn(ClO4)6] + 4 ClO2 + 2 Cl2
It reacts with gold to produce the chloryl salt [ClO2]+[Au(ClO4)4]−:[7]
- 2 Au + 6 Cl2O6 → 2 [ClO2]+[Au(ClO4)4]− + Cl2
Several other transition metal perchlorate complexes are prepared using dichlorine hexoxide.
Nevertheless, it can also react as a source of the ClO3 radical:[citation needed]
- 2 AsF5 + Cl2O6 → 2 ClO3AsF5
Remove ads
Synthesis
- 4 ClO2 + 2 O3 → 2 Cl2O6 + O2 (under ultraviolet light)
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads