Top Qs
Timeline
Chat
Perspective
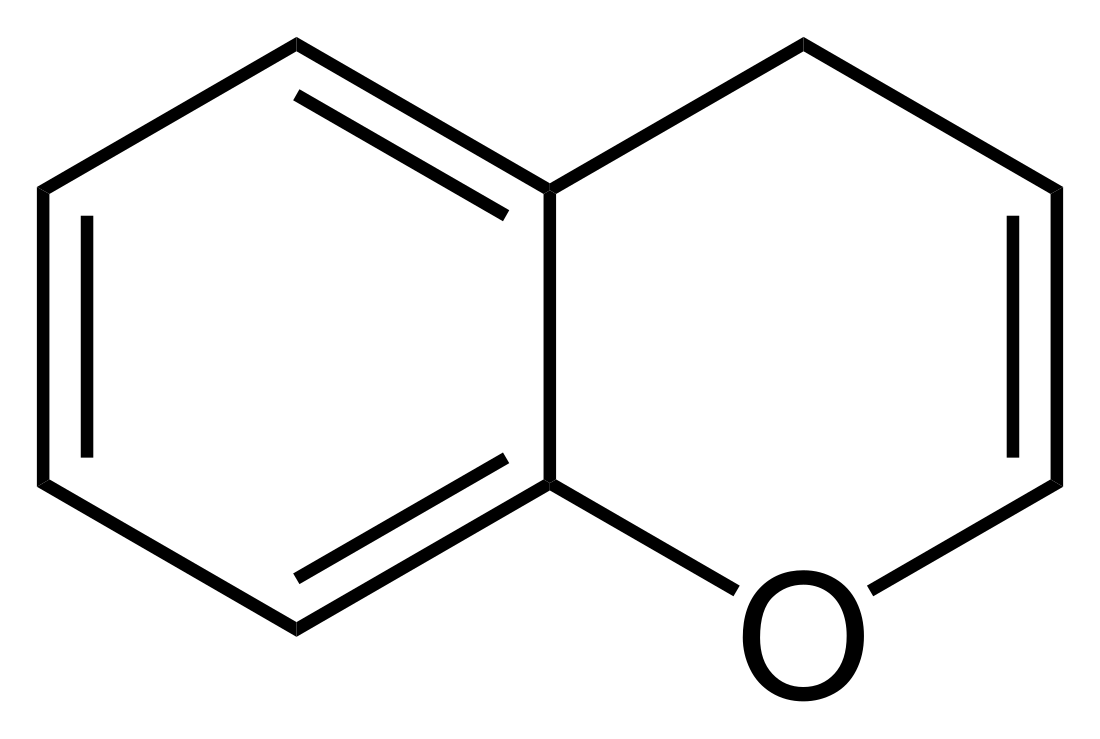
4H-1-Benzopyran
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
4H-1-Benzopyran is an organic compound with the formula C6H4C3H4O. It is one of two isomers of benzopyran, the other being 2H-1-benzopyran, which is more prevalent. It can be viewed as the fusion of a benzene ring to a heterocyclic pyran ring.[1]
This article needs additional citations for verification. (January 2017) |
Some benzopyrans have shown anticancerous activity in vitro.[2]
The radical form of benzopyran is paramagnetic. The unpaired electron is delocalized over the whole benzopyran molecule, rendering it less reactive than one would expect otherwise. A similar example is the cyclopentadienyl radical. Commonly, benzopyran is encountered in the reduced state, in which it is partially saturated with one hydrogen atom, introducing a tetrahedral CH2 group in the pyran ring. Therefore, there are many structural isomers owing to the multiple possible positions of the oxygen atom and the tetrahedral carbon atom:
 2H-chromene (2H-1-benzopyran) |
 4H-chromene (4H-1-benzopyran) |
| 5H-chromene | 7H-chromene |
| 8aH-chromene |
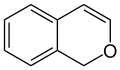 1H-isochromene (1H-2-benzopyran) |
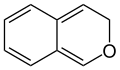 3H-isochromene (3H-2-benzopyran) |
Remove ads
Nomenclature
According to current IUPAC nomenclature, the name chromene used in previous recommendations is retained; however, systematic ‘benzo’ names, for example 2H-1-benzopyran, are preferred IUPAC names for chromene, isochromene, chromane, isochromane, and their chalcogen analogues.[3] There are two isomers of benzopyran that vary by the orientation of the fusion of the two rings compared to the oxygen, resulting in 1-benzopyran (chromene) and 2-benzopyran (isochromene)—the number denotes where the oxygen atom is located by standard naphthalene-like nomenclature.
Remove ads
See also
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads