Top Qs
Timeline
Chat
Perspective
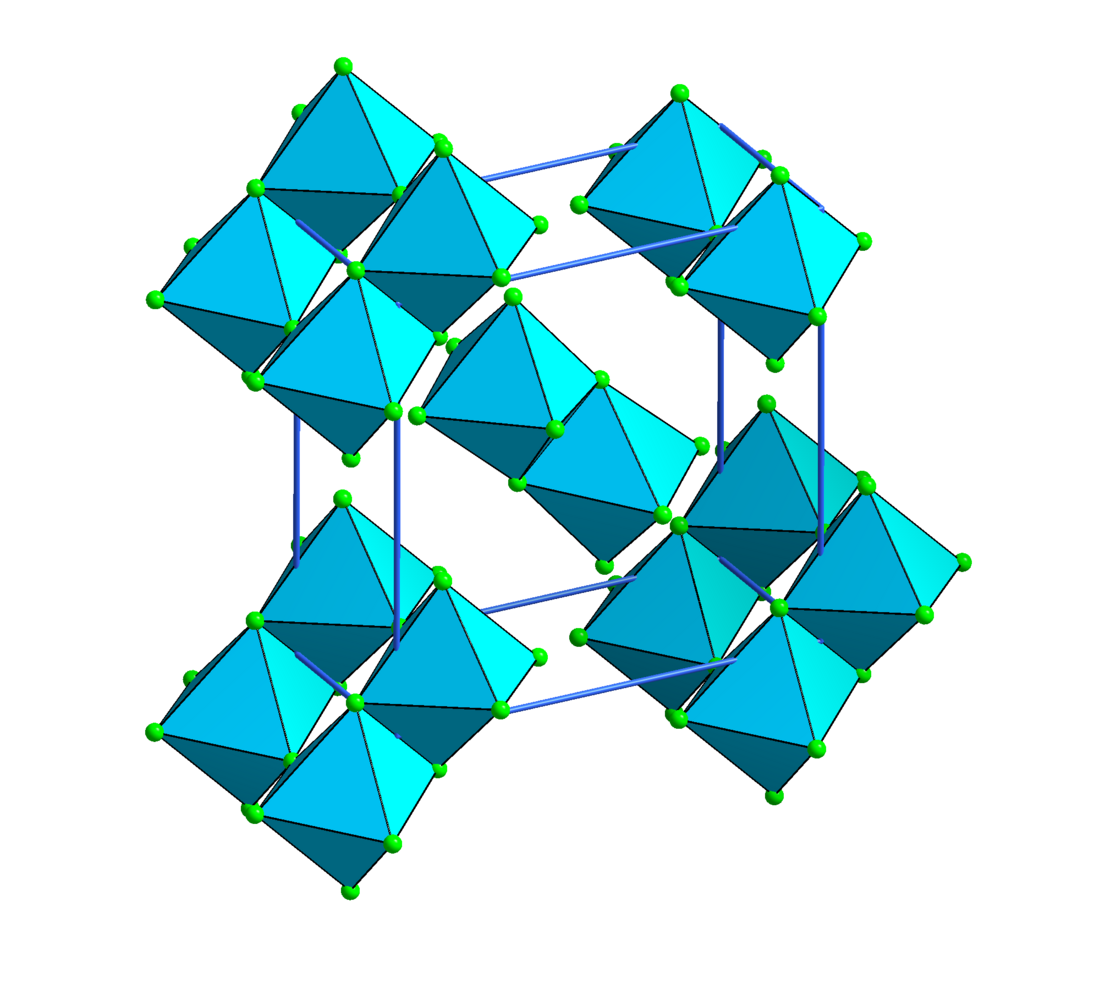
Chromium(IV) fluoride
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Chromium(IV) fluoride is an inorganic compound with the chemical formula CrF4. It has a dark greenish-black color when solid. It rapidly hydrolyzes in presence of moisture in air or directly in water.[3]
Remove ads
Synthesis
Powdered chromium or CrCl3 is exposed to fluorine gas at a temperature of 350-500 °C, which creates a mix of CrF4 and CrF5. The CrF4 settles out as varnish-like brown beads upon cooling.[2]
Reactions
Chromium(IV) fluoride is easily reduced.[4]
It will react with water:
- CrF4 + 2H2O → CrO2 + 4HF
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads