Top Qs
Timeline
Chat
Perspective
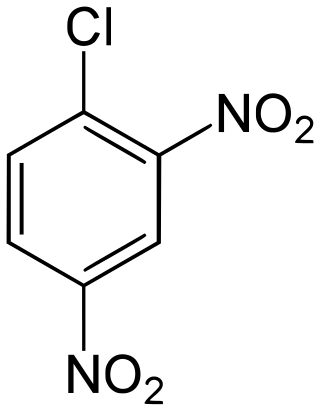
2,4-Dinitrochlorobenzene
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
2,4-Dinitrochlorobenzene (DNCB) is an organic compound with the chemical formula (O2N)2C6H3Cl. It is a yellow solid that is soluble in organic solvents. It is an intermediate for the industrial production of other compounds.[2]
Remove ads
Preparation and reactions
DNCB is produced commercially by the nitration of p-nitrochlorobenzene with a mixture of nitric and sulfuric acids. Other methods afford the compound less efficiently include the chlorination of 1,3-dinitrobenzene, nitration of o-nitrochlorobenzene and the dinitration of chlorobenzene.[3]
By virtue of the two nitro substituents, the chloride in DNCB is particularly susceptible to nucleophilic substitution, at least relative to simple chlorobenzene. In this way, the compound is a precursor to many other compounds. For example, the chloride can be replaced by iodide easily.[4][5][6]
Reaction of DNCB with ammonia gives 2,4-dinitrochloroaniline, again a versatile precursor.[2]
DNCB is as a substrate in glutathione S-transferase, relevant to activity assays.[7]
Remove ads
Safety
DNCB induces a type IV hypersensitivity reaction in almost all people exposed to it, so it is used medically to assess the T cell activity in patients. This is a useful diagnostic test for immunocompromised patients. It can also be used to treat warts.[8]
DNCB can cause contact dermatitis.[9]
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads