Top Qs
Timeline
Chat
Perspective
Iron(II) lactate
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
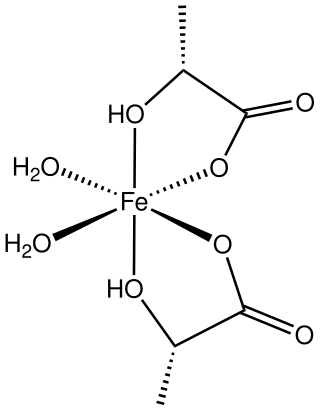
Ferrous lactate, or iron(II) lactate, are coordination complexes of iron(II) with one or more lactate ligands. One example is Fe(lactate)2(H2O)2(H2O) where lactate is CH3CH(OH)CO−2.[2] It is a colorless solid.
Remove ads
Production
Iron(II) lactate can be produced through several reactions, among which are calcium lactate with iron(II) sulfate according to the following reaction:[3]
- Ca(C3H5O3)2(aq) + FeSO4(aq) → CaSO4↓ + Fe(C3H5O3)2(aq)
Another route yielding iron(II) lactate is to combine lactic acid with calcium carbonate and iron(II) sulfate.
Uses
Iron (II) lactate is used as a reagent in the production of proton-exchange membrane fuel cells (PEMFCs), specifically in the production of cathode catalytic converters used in these cells. It is an acidity regulator and, since it oxidizes on contact with air, it has found use as a color retention agent for foodstuffs such as olives. It is also used to fortify foods with iron, as a remedy for anemia due to iron deficiency, and as a nutritional supplement in tablet or pill form. As a food additive it is coded under the E number E585.[citation needed]
Remove ads
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads