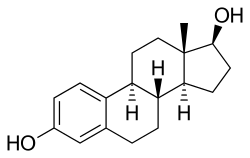
Estradiol/medroxyprogesterone acetate (E2/MPA), sold under the brand names Indivina and Tridestra among others, is a combination product of estradiol, an estrogen, and medroxyprogesterone acetate, a progestogen, which is used in menopausal hormone therapy for the treatment of menopausal symptoms.[1] It is taken by mouth.
More information Clinical outcome, Hypothesized effect on risk ...
Results of the Women's Health Initiative (WHI) menopausal hormone therapy randomized controlled trials
| Clinical outcome |
Hypothesized
effect on risk |
Estrogen and progestogen
(CEsTooltip conjugated estrogens 0.625 mg/day p.o. + MPATooltip medroxyprogesterone acetate 2.5 mg/day p.o.)
(n = 16,608, with uterus, 5.2–5.6 years follow up) |
Estrogen alone
(CEsTooltip Conjugated estrogens 0.625 mg/day p.o.)
(n = 10,739, no uterus, 6.8–7.1 years follow up) |
|---|
| HRTooltip Hazard ratio |
95% CITooltip Confidence interval |
ARTooltip Attributable risk |
HRTooltip Hazard ratio |
95% CITooltip Confidence interval |
ARTooltip Attributable risk |
| Coronary heart disease |
Decreased |
1.24 |
1.00–1.54 |
+6 / 10,000 PYs |
0.95 |
0.79–1.15 |
−3 / 10,000 PYs |
| Stroke |
Decreased |
1.31 |
1.02–1.68 |
+8 / 10,000 PYs |
1.37 |
1.09–1.73 |
+12 / 10,000 PYs |
| Pulmonary embolism |
Increased |
2.13 |
1.45–3.11 |
+10 / 10,000 PYs |
1.37 |
0.90–2.07 |
+4 / 10,000 PYs |
| Venous thromboembolism |
Increased |
2.06 |
1.57–2.70 |
+18 / 10,000 PYs |
1.32 |
0.99–1.75 |
+8 / 10,000 PYs |
| Breast cancer |
Increased |
1.24 |
1.02–1.50 |
+8 / 10,000 PYs |
0.80 |
0.62–1.04 |
−6 / 10,000 PYs |
| Colorectal cancer |
Decreased |
0.56 |
0.38–0.81 |
−7 / 10,000 PYs |
1.08 |
0.75–1.55 |
+1 / 10,000 PYs |
| Endometrial cancer |
– |
0.81 |
0.48–1.36 |
−1 / 10,000 PYs |
– |
– |
– |
| Hip fractures |
Decreased |
0.67 |
0.47–0.96 |
−5 / 10,000 PYs |
0.65 |
0.45–0.94 |
−7 / 10,000 PYs |
| Total fractures |
Decreased |
0.76 |
0.69–0.83 |
−47 / 10,000 PYs |
0.71 |
0.64–0.80 |
−53 / 10,000 PYs |
| Total mortality |
Decreased |
0.98 |
0.82–1.18 |
−1 / 10,000 PYs |
1.04 |
0.91–1.12 |
+3 / 10,000 PYs |
| Global index |
– |
1.15 |
1.03–1.28 |
+19 / 10,000 PYs |
1.01 |
1.09–1.12 |
+2 / 10,000 PYs |
| Diabetes |
– |
0.79 |
0.67–0.93 |
|
0.88 |
0.77–1.01 |
|
| Gallbladder disease |
Increased |
1.59 |
1.28–1.97 |
|
1.67 |
1.35–2.06 |
|
| Stress incontinence |
– |
1.87 |
1.61–2.18 |
|
2.15 |
1.77–2.82 |
|
| Urge incontinence |
– |
1.15 |
0.99–1.34 |
|
1.32 |
1.10–1.58 |
|
| Peripheral artery disease |
– |
0.89 |
0.63–1.25 |
|
1.32 |
0.99–1.77 |
|
| Probable dementia |
Decreased |
2.05 |
1.21–3.48 |
|
1.49 |
0.83–2.66 |
|
Close
Quick facts Combination of, Medroxyprogesterone acetate ...
Close