Top Qs
Timeline
Chat
Perspective
Europium(II) iodide
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Europium(II) iodide is the iodide salt of divalent europium cation.
Remove ads
Preparation
Europium(II) iodide can be prepared in a handful of ways, including:
Reduction of europium(III) iodide with hydrogen gas at 350 °C:[1]
- 2 EuI3 + H2 → 2 EuI2 + 2 HI
Thermal decomposition of europium(III) iodide at 200 °C:[1]
- 2 EuI3 → 2 EuI2 + 2 I2
Reaction of europium with mercury(II) iodide:[1]
- Eu + HgI2 → Eu I2 + Hg
Reaction of europium with ammonium iodide:[1]
- Eu + 2 NH4I → EuI2 + 2 NH3 + H2
Remove ads
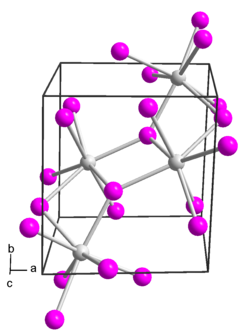
Structure
Europium(II) iodide has several polymorphs.[2] It adopts a monoclinic crystal structure in space group P 21/c (no. 14).[3][4]
It also adopts an orthorhombic polymorph in space group Pbca (no. 61). This form is isostructural with strontium iodide.[5]
A third polymorph of europium(II) iodide is formed if it is prepared from europium and ammonium iodide at low temperatures (200 K) in liquid ammonia. This low-temperature phase is orthorhombic and in space group Pnma (no. 62). This is the same structure as modification IV of strontium iodide.[6]
Remove ads
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads