Top Qs
Timeline
Chat
Perspective
Gomberg's dimer
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
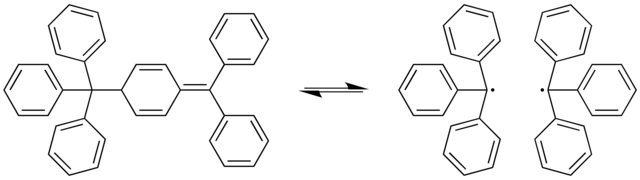
Gomberg's dimer is the organic compound with the formula Ph2C=C6H5-CPh3, where Ph = C6H5. It is a yellow solid that is air-stable for hours at room temperature and soluble in organic solvents.[1] The compound achieved fame as the dimer of triphenylmethyl radical, which was prepared by Moses Gomberg in his quest for hexaphenylethane.
Its quinoid structure has been determined by X-ray crystallography. The C-C bond that reversibly breaks is rather long at 159.7 picometers.[1]
Remove ads
Synthesis and reactions
Gomberg's dimer can be prepared quantitatively by treating trityl bromide with powdered copper or silver:[2]
- 2 Ph3CBr + 2 Cu → Ph2C=C6H5-CPh3 + 2 CuBr
Gomberg's dimer reversibly dissociates to the triphenylmethyl radical in organic solvents:[3]
See also
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads