Top Qs
Timeline
Chat
Perspective
Heme B
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
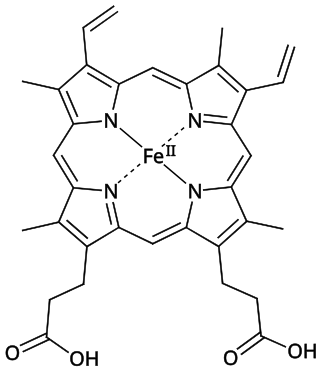
Heme B or haem B (also known as protoheme IX) is the most abundant heme.[1] Hemoglobin and myoglobin are examples of oxygen transport proteins that contain heme B. The peroxidase family of enzymes also contain heme B. The COX-1 and COX-2 enzymes (cyclooxygenase) of recent fame, also contain heme B at one of two active sites. Isolated heme B is slightly soluble in ammonia water and is extremely sensitive to oxygen, instantly oxidizing to a dark green to black-brown color when exposed to air.
This scientific article needs additional citations to secondary or tertiary sources. (December 2025) |
Generally, heme B is attached to the surrounding protein matrix (known as the apoprotein) through a single coordination bond between the heme iron and an amino-acid side-chain.
Both hemoglobin and myoglobin have a coordination bond to an evolutionarily-conserved histidine, while nitric oxide synthase and cytochrome P450 have a coordination bond to an evolutionarily-conserved cysteine bound to the iron center of heme B.
Since the iron in heme B containing proteins is bound to the four nitrogens of the porphyrin (forming a plane) and a single electron donating atom of the protein, the iron is often in a pentacoordinate state. When oxygen or the toxic carbon monoxide is bound the iron becomes hexacoordinated. The correct structures of heme B and heme S were first elucidated by German chemist Hans Fischer.[2]
Remove ads
Chemical properties
(Suspended)
These are four concentrated ammonia water solutions. From left to right: nicotinamide adduct of Heme B, carbon monoxide adduct of Heme B, nicotinic acid adduct of Heme B, and Heme B. They are slightly soluble in water and are all highly sensitive to oxygen in the air, requiring oxygen-free storage.
These are four concentrated ammonia water solutions. From left to right: nicotinamide adduct of Heme B, carbon monoxide adduct of Heme B, nicotinic acid adduct of Heme B, and Heme B. They are slightly soluble in water and are all highly sensitive to oxygen in the air, requiring oxygen-free storage.
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads