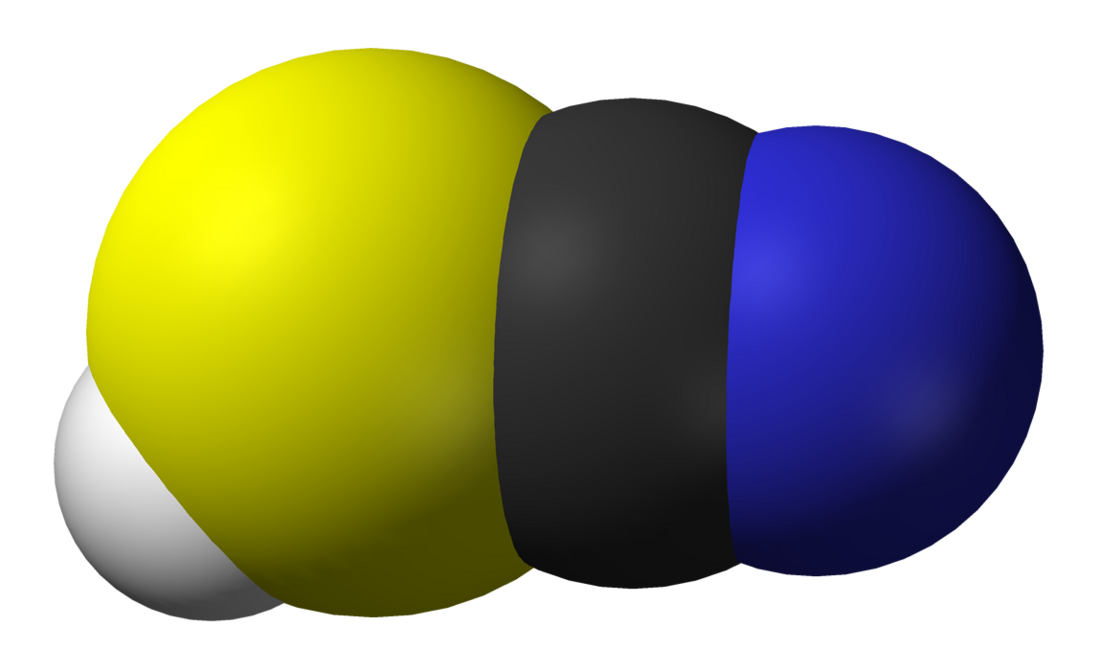Top Qs
Timeline
Chat
Perspective
Thiocyanic acid
Chemical compound (H–S–C≡N) From Wikipedia, the free encyclopedia
Remove ads
Thiocyanic acid is a chemical compound with the formula HSCN and structure H−S−C≡N, which exists as a tautomer with isothiocyanic acid (H−N=C=S).[8] The isothiocyanic acid tautomer tends to dominate with the compound being about 95% isothiocyanic acid in the vapor phase.[9]
It is a moderately strong acid,[10] with a pKa of 1.1 at 20 °C and extrapolated to zero ionic strength.[11]
One of the thiocyanic acid tautomers, HSCN, is predicted to have a triple bond between carbon and nitrogen. Thiocyanic acid has been observed spectroscopically.[12]
The salts and esters of thiocyanic acid are known as thiocyanates. The salts are composed of the thiocyanate ion ([SCN]−) and a suitable cation (e.g., potassium thiocyanate, KSCN). The esters of thiocyanic acid have the general structure R−S−C≡N, where R stands for an organyl group.
Isothiocyanic acid, HNCS, is a Lewis acid whose free energy, enthalpy and entropy changes for its 1:1 association with a variety of Lewis bases in carbon tetrachloride solution at 25 °C have been reported.[13]< HNCS acceptor properties are discussed in the ECW model. The salts are composed of the thiocyanate ion ([SCN]−) and a suitable cation (e.g., ammonium thiocyanate, [NH4]+[SCN]−). Isothiocyanic acid forms isothiocyanates R−N=C=S, where R stands for an organyl group.
Thiocyanuric acid is a stable trimer of thiocyanic acid.
Wikimedia Commons has media related to Thiocyanic acid.
Remove ads
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads