Top Qs
Timeline
Chat
Perspective
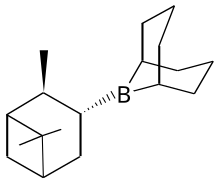
Alpine borane
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Alpine borane is the commercial name for an organoboron compound used in organic synthesis. It is a colorless liquid, which is usually encountered as a solution. A range of alkyl-substituted boranes are specialty reagents in organic synthesis. Two such reagents closely related to Alpine borane are 9-BBN and diisopinocampheylborane.
Remove ads
Preparation and reactions
This reagent is generated by treating 9-BBN with α-pinene.[2]
This sterically crowded chiral trialkylborane can stereoselectively reduce aldehydes in what is known as the Midland Alpine borane reduction, or simply the Midland reduction.[3]
- C8H12B-pinanyl + RCDO → C8H12BOCHDR + (+)-d-pinene
Hydrolysis of the resulting borinic ester affords the alcohol:
- C8H12BOCHDR + H2O → C8H12BOH + HOCHDR
It is also effective for the stereoselective reduction of certain acetylenic ketones.[4] The reaction proposed involves forming an adduct by coordinating the carbonyl oxygen to boron. Intramolecular hydride transfer from the pinane substituent to the carbonyl carbon ensues. Many substrates for the Midland reduction have a low steric group, such as an alkyne[5] or a nitrile,[6] to increase selectivity. Stereochemical control comes from the coordination of the carbonyl bulky borane, followed by hydride transfer opposite the largest group.[2][7]

Remove ads
See also
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads