Top Qs
Timeline
Chat
Perspective
Neptunium sulfides
Compounds of neptunium and sulfur From Wikipedia, the free encyclopedia
Remove ads
Neptunium sulfides are compounds of neptunium and sulfur. In these compounds, neptunium has an oxidation state of +3 or +4, and sulfur exists as sulfide or polysulfide ions. They have the general formula NpxSy. Known neptunium sulfides include NpS, Np3S4, Np2S3, Np3S5, NpS2, Np2S5, and NpS3. These compounds are often isostructural with their corresponding uranium or plutonium compounds. Neptunium oxysulfides (mixed oxide-sulfides) are also known, including Np2O2S, Np4O4S3, and NpOS.[1][2][3]
Remove ads
Neptunium(III) sulfides
Summarize
Perspective
Neptunium monosulfide
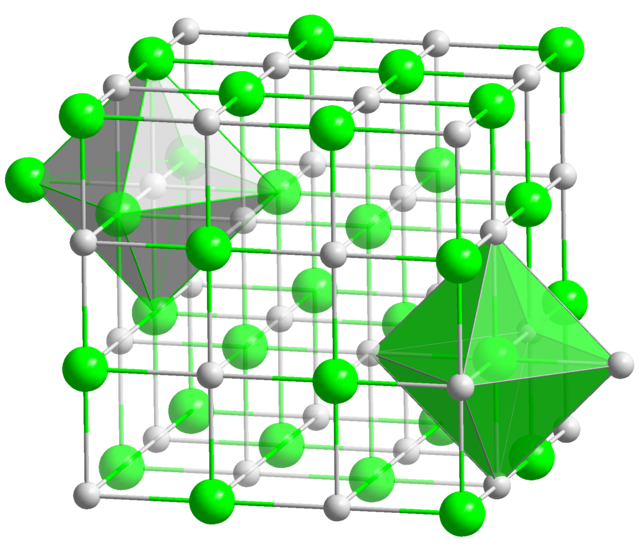
Neptunium monosulfide has the formula NpS. It features neptunium in the +3 state, being an electride salt like plutonium monosulfide. Like plutonium monosulfide, it adopts a rock salt structure, with lattice constant a=5.532 Å.[3][4]
It can be produced by reducing Np2S3 with neptunium metal at 1600 °C:[1]
- Np2S3 + Np → 3 NpS
It can also be produced by reacting neptunium metal with sulfur gas:[1]
- Np + S → NpS
It behaves as a resistor, with an electrical resistivity of 60,000 μΩ⋅cm.[4]
It is predicted to undergo a phase transition to a caesium chloride-type structure at 75 GPa, with a 3.7% volume loss from the NaCl-type structure.[5]
Neptunium sesquisulfide

Neptunium sesquisulfide has the formula Np2S3. It has several polymorphs, which are isostructural with the corresponding plutonium sulfides. It is often hypostoichiometric, with compositions ranging between Np3S4, Np5S7, and Np2S3.[1][2][4]
It was first prepared by reacting neptunium dioxide with carbon disulfide and hydrogen sulfide in 1948. It can also be prepared via thermal composition of Np3S5 at ~1200 K in vacuum:[1][4]
- 2 Np3S5 → 3 Np2S3 + S
Np2S3 was initially reported to be isostructural with uranium sesquisulfide (dubbed η-Np2S3) with lattice parameters a=10.3, b=10.6, and c=3.9 Å. However, later experiments could not reproduce these results.[1][2][4]
Later experiments[2][4] found evidence for three polymorphs of Np2S3: α-, β-, and γ-Np2S3. These are isostructural with the corresponding plutonium compounds.[1]
α-Np2S3 is the form of Np2S3 present at standard temperature. It has an α-Ce2S3-type structure. Unlike β-Np2S3 and γ-Np2S3, it is stoichiometric. It is orthorhombic with lattice parameters a=3.98, b=7.39, and c=15.50 Å.[1]
β-Np2S3 can be formed from heating α-Np2S3 to around ~1500 K. It has a β-Ce2S3-type structure, being tetragonal with lattice parameters a=14.94, b=7.39, and c=19.84 Å. It has a stoichiometry between Np5S7 and Np2S3-ε.[4]
γ-Np2S3 can be formed from heating β-Np2S3 to around ~1800 K. It adopts the cubic Th3P4-type structure, being cubic with lattice parameter a=8.440 Å. It has a stoichiometry between Np3S4 and Np2S3-ε.[1][2][4]
Remove ads
Neptunium(III,IV) sulfides
Trineptunium pentasulfide

Trineptunium pentasulfide has the formula Np3S5. Like the corresponding neptunium selenide, Np3Se5, it is antiferromagnetic, undergoing magnetic ordering at 35 K. It is a black solid which is isostructural with triuranium pentasulfide.[3]
It can be obtained by the thermal decomposition of neptunium trisulfide at 500 °C or by reacting neptunium and sulfur in caesium chloride flux.[1][3][4]
It decomposes into α-Np2S3 at 900 °C. At normal temperatures, however, it is highly stable, and is a common byproduct of reactions involving compounds of neptunium and sulfur.[3][4]
It contains NpIII and NpIV ions in a 2:1 ratio, and its formula can be represented as (Np3+)2(Np4+)(S2−)5.[3]
Remove ads
Neptunium(IV) sulfides
Neptunium disulfide
Neptunium disulfide has the formula NpS2. Very little information about it is available, and it is difficult to synthesize. Mössbauer spectroscopy indicates that it contains NpIV.[4][6]
Dineptunium pentasulfide

Dineptunium pentasulfide has the formula Np2S5. It is isostructural with the corresponding thorium sulfide (Th2S5) and uranium sulfide (U2S5). It is a polysulfide, and its formula can be represented as (Np4+)2(S2−)3(S2−2). It forms tetragonal crystals, with lattice parameters a=10.48 and c=9.84 Å. It can be synthesized by reacting Np3S5 with sulfur at 500 °C: [1][4]
- 2 Np3S5 + 5 S → 3 Np2S5
Neptunium trisulfide
Neptunium trisulfide has the formula NpS3. It has been found to undergo magnetic ordering at low temperatures (~45 K). It is a polysulfide, and its formula can be represented as (Np4+)(S2−)(S2−2).[1][2]
It can be formed via the reaction of neptunium and sulfur at 500 °C:
- Np + 3 S → NpS3
It has a monoclinic structure, isostructural with US3, with lattice parameters a=5.36, b=3.87, c=18.10 Å, and β=99°.[4]
Neptunium oxysulfides
Np2O2S
Dineptunium dioxide monosulfide (Np2O2S) is the only stable oxysulfide of neptunium at high temperatures (~1600 °C). It is isostructural with other lanthanide and actinide oxysulfides, like La2O2S, having a hexagonal crystal structure with lattice parameters a=3.95 and c=6.80 Å. It is formed by the high temperature decomposition of Np4O4S3. It contains NpIII.[1][4]
Np4O4S3
Tetraneptunium tetroxide trisulfide (Np4O4S3) is formed from the decomposition of NpOS in vacuum at 700 °C. It is isostructural with Pu4O4S3, having a pseudo-hexagonal structure with lattice parameters a=4.07, b=6.76, c=3.89 Å, and β=118°. It contains NpIII and NpIV in equal amounts. Its formula can be written as (Np3+)2(Np4+)2(O2−)4(S2−)3.[4]
NpOS
Neptunium monoxide monosulfide (NpOS) is commonly encountered as a result of oxidation of other neptunium sulfides, e.g. Np3S5. Pure NpOS can be formed by oxidizing NpS in a sealed ampoule at 700 °C:[3][4]
- 2 NpS + O2 → 2 NpOS
It is isostructural with the corresponding uranium oxysulfide and plutonium oxysulfide, forming tetragonal crystals with lattice parameters a=3.815 and c=6.623 Å. It contains NpIV.[4]
Remove ads
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads