Top Qs
Timeline
Chat
Perspective
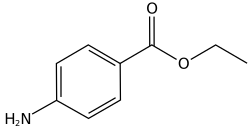
Benzocaine
Local anaesthetic drug From Wikipedia, the free encyclopedia
Remove ads
Benzocaine, sold under the brand name Orajel amongst others, is a local anesthetic, belonging to the amino ester drug class, commonly used as a topical painkiller or in cough drops. It is the active ingredient in many over-the-counter anesthetic ointments such as products for oral ulcers. It is combined with antipyrine to form A/B ear drops. In the US, products containing benzocaine for oral application are contraindicated in children younger than two years old.[2][3]
It was first synthesised in 1890 in Germany and approved for medical use in 1902.[4]
Remove ads
Medical uses
Summarize
Perspective
Benzocaine is indicated to treat a variety of pain-related conditions. It may be used for:
- Local anesthesia of oral and pharyngeal mucous membranes (sore throat, cold sores, mouth ulcers, toothache, sore gums, denture irritation)[5]
- Otic pain (earache)[5]
- Surgical or procedural local anesthesia[6]
- Relief of skin pain caused by sunburn, ingrown toenails, hemorrhoids,[7]
Examples of combination medications of benzocaine include:
- Antipyrine-benzocaine otic consists of antipyrine and benzocaine, and is used to relieve ear pain and remove earwax.[8]
- Cepacol consists of menthol and benzocaine, and is used to treat sore throat.[9]
- A solution of benzocaine and menthol is marketed for the treatment of bee stings, mosquito bites, jellyfish stings, and other insect bites[10]
Other uses

Benzocaine is used as a key ingredient in numerous pharmaceuticals:
- Some glycerol-based ear medications for use in removing excess wax as well as relieving ear conditions such as otitis media and swimmer's ear.
- Some previous diet products such as Ayds.
- Some condoms designed to prevent premature ejaculation. Benzocaine largely inhibits sensitivity on the penis, and can allow for an erection to be maintained longer (in a continuous act) by delaying ejaculation. Conversely, an erection will also fade faster if stimulus is interrupted.[11][12]
- Benzocaine mucoadhesive patches have been used in reducing orthodontic pain.[13]
- In Poland it is included, together with menthol and zinc oxide, in the liquid powder (not to be confused with the liquid face powder) used mainly after mosquito bites. Today's ready-made Pudroderm[14] was once used there as pharmaceutical compound.
Available forms
Benzocaine can come in a variety of preparations including:
Oral preparations:
- Lozenges (ex. Cepacol, Mycinettes)[15][failed verification]
- Throat Spray (ex. Ultra Chloraseptic)[16]
Topical preparations:
- Aerosol (ex. Topex)[17][failed verification]
- Gel (ex. Orajel, Kank-A)[18][failed verification] [19]
- Paste (ex. Orabase)[20][failed verification]
- Cream (ex. Lanacane - active ingredient 3% Benzocaine)
Otic preparations:
- Solution (ex. Allergen)
Remove ads
Side effects
Benzocaine is generally well tolerated and non-toxic when applied topically as recommended.[21]
However, there have been reports of serious, life-threatening adverse effects (e.g., seizures, coma, irregular heart beat, respiratory depression) with over-application of topical products or when applying topical products that contain high concentrations of benzocaine to the skin.[22]
The topical use of higher concentration (10–20%) benzocaine products applied to the mouth or mucous membranes has been found to be a cause of methemoglobinemia, a disorder in which the amount of oxygen carried by the blood is greatly reduced.[23]
Benzocaine may cause allergic reactions.[24][25][26][27] These include:
- Contact dermatitis (redness and itchiness)[28]
- Anaphylaxis (rare)[28]
Remove ads
Chemistry
Benzocaine is the ethyl ester of p-aminobenzoic acid (PABA). It can be prepared from PABA and ethanol[29] by Fischer esterification or via the reduction of ethyl p-nitrobenzoate. Benzocaine is sparingly soluble in water; it is more soluble in dilute acids and very soluble in ethanol, chloroform, and ethyl ether. The melting point of benzocaine is 88–92 °C,[30] and the boiling point is about 310 °C.[31] The density of benzocaine is 1.17 g/cm3.
Synthesis
Benzocaine can be prepared by esterification using 4-aminobenzoic acid and ethanol.[32][33] It can also be prepared by reduction of ethyl 4-nitrobenzoate to the amine.[34][35] In industrial practice, the reducing agent is usually iron and water in the presence of a little acid.[36]
History
Benzocaine was first synthesized in 1890 by the German chemist Eduard Ritsert (1859–1946),[37] in the town of Eberbach[38] and introduced to the market in 1902 under the name "Anästhesin".[39][40]
Society and culture
Benzocaine is found, particularly in Britain, as an additive in street cocaine and also as a bulking agent in "legal highs".[41] Benzocaine gives a numbing effect similar to cocaine and as a bulking and binding agent it can not be detected once mixed. It is the most popular cutting agent worldwide.[42]
Veterinary uses
Bath solutions of benzocaine and its derivatives are commonly used to anesthetize amphibians for surgery.[43][44] Benzocaine-based anesthetics are potent and highly effective for both anesthesia and euthanasia in amphibians.[45]
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads