Top Qs
Timeline
Chat
Perspective
Organolithium chemistry
Science of organotitanium compounds From Wikipedia, the free encyclopedia
Remove ads
Organolithium chemistry is the science of organolithium compounds describing their physical properties, synthesis, and reactions. Organolithium compounds in organometallic chemistry contain carbon-lithium chemical bonds. A major subset and perhaps the most used organolithium compounds are organolithium reagents, such as butyllithium[1]
Classification
Summarize
Perspective
Alkyl and aryl monolithium compounds
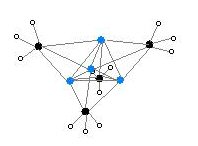

 Various depictions of the methyl lithium tetramer. Colour code: Li- purple C- black H- white
Various depictions of the methyl lithium tetramer. Colour code: Li- purple C- black H- white
Alkyl and aryl monolithium are illustrated by their simplest members, methyl lithium and phenyl lithium. These useful reagents have complicated structures owing to the tendency of the C-Li unit to aggregate, forming clusters.[2] These compounds are prepared by metal-halogen exchange, which entails stirring solutions of the alkyl halide with lithium metal:
- CH3Br + Li → CH3Li + LiBr
Lithium-reduced polycyclic aromatics

Naphthalene, anthracene, and related polycyclic aromatic compounds are reduced by lithium to give Li+ salts of their radical anions:[3]
- C10H8 + Li → Li+C10H−8
Lithium naphthalene (Li+[C10H8]−) is used as a reductant in the synthesis of organic, organometallic, and inorganic chemistry.
Dilithio compounds
These compounds, which have two C-Li bonds can be classified into two distinct groups. Simplest but rare is dilithiomethanide (CH2Li2) and its many derivatives.[4] Some derivatives have even been characterized by X-ray crystallography.[5]
The more common dilithiated organic compounds the Li atoms are bound to separate carbon atoms. 1,4-dilithiobutane (LiCH2CH2CH2CH2Li) would be an example.
Dilithioacetylide (Li2C2, again is not a salt, but adopts a distorted anti-fluorite crystal structure, similar to that of rubidium peroxide (Rb2O2).[6]
Tetralithio compounds
Tetralithiomethane has attracted attention mainly for theoretical reasons. Its structure remains uncertain, but some speculation posited planar carbon.
Remove ads
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads