Top Qs
Timeline
Chat
Perspective
Chloranil
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
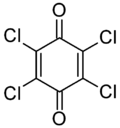
Chloranil is a quinone with the molecular formula C6Cl4O2. Also known as tetrachloro-1,4-benzoquinone, it is a yellow solid. Like the parent benzoquinone, chloranil is a planar molecule[2] that functions as a mild oxidant.
Remove ads
Synthesis and use as reagent
Chloranil is produced by chlorination of phenol to give hexachlorocyclohexa-2,5-dien-1-one ("hexachlorophenol"). Hydrolysis of the dichloromethylene group in this dienone gives chloranil:[3]
- C6H5OH + 6 Cl2 → C6Cl6O + 6 HCl
- C6Cl6O + H2O → C6Cl4O2 + 2 HCl
Chloroanil serves as a hydrogen acceptor. It is more electrophilic than quinone itself. It is used for the aromatization reactions, such as the conversion of cyclohexadienes to the benzene derivatives.[4]
Chloranil is used to test for free secondary amines. This test is useful for checking for the presence of proline derivatives. It is also a good test for the successful deprotection of a secondary amine. Secondary amines react with chloranil to give a brown/red/orange derivative, the colour depending on the amine. In these reactions, the amine displaces chloride from the ring of the quinone.
Remove ads
Commercial applications
It is a precursor to many dyes, such as pigment violet 23 and diaziquone (AZQ), a cancer chemotherapeutic agent.
See also
References
External links
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads