Top Qs
Timeline
Chat
Perspective
Palladium(II) iodide
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Palladium(II) iodide is an inorganic compound of palladium and iodine. It is commercially available, though less common than palladium(II) chloride, the usual entry point to palladium chemistry. Three polymorphs are known.[2]
Remove ads
Preparation
Palladium(II) iodide can be obtained by treating a dilute solution of palladium in nitric acid with sodium iodide at 80 °C.[2]
The high-temperature polymorph α-palladium(II) iodide can be produced by reaction of the elements at temperature above 600 °C. The γ-modification is produced as an almost amorphous powder by addition of iodide salts to aqueous H2PdCl4 solution . When heated in dilute hydrogen iodide solution, this polymorph transforms into the β phase at around 140 °C.[3]
Remove ads
Reactions and uses
Palladium(II) iodide is insoluble in water. It reacts with iodide giving PdI42− anion:
- PdI2 + 2I− → PdI2−4
It finds use as a catalyst.[4]
Historically, the quantity of palladium in a solution may be determined gravimetrically by precipitation as palladium(II) iodide.[5]
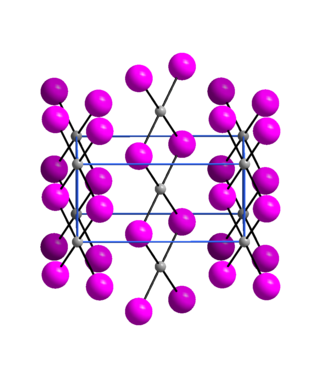
Crystallography
Palladium(II) iodide is an almost X-ray amorphous black powder. The α-modification has an orthorhombic crystal structure with the space group Pnmn(space group no. 58, position 5).[6]
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads