Top Qs
Timeline
Chat
Perspective
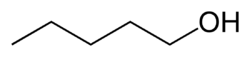
1-Pentanol
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
1-Pentanol, (or n-pentanol, pentan-1-ol), is an organic compound with the formula CH3CH2CH2CH2CH2OH and is classified as a primary alcohol.[2] It is a colourless liquid with a distinctive aroma. It is one of 8 isomeric alcohols with the formula C5H11OH. It is used as a solvent, a biological drying agent and in the synthesis of some fragrance compounds. It is also a common component of fusel alcohols (fusel oils), the undesirable byproducts of alcoholic fermentation.
Remove ads
Preparation
1-Pentanol is prepared from 1-butene by hydroformylation followed by hydrogenation of the resulting pentanal.[3]
- CH3CH2CH=CH2 + CO + H2 → CH3CH2CH2CH2CHO
- CH3CH2CH2CH2CHO + H2 → CH3CH2CH2CH2CH2OH
Pentanol can be prepared by fractional distillation of fusel oil. To reduce the use of fossil fuels, research is underway to develop cost-effective methods of producing (chemically identical) bio-pentanol with fermentation.[4][5]
Remove ads
Uses and occurrence
The hydroxyl group (OH) is the active site of many reactions. The ester formed from 1-pentanol and butyric acid is pentyl butyrate, which has an apricot-like odor. The ester formed from 1-pentanol and acetic acid is amyl acetate (also called pentyl acetate), which has a banana-like odor.
It is a precursor to dipentyl zinc dithiophosphates, which are used in froth flotation.[3]
In 2014, a study was conducted comparing the performance of diesel fuel blends with various proportions of pentanol as an additive. While gaseous emissions increased with higher concentrations of pentanol, particulate emissions decreased.[6]
Pentanol is often used as a solvent.
Remove ads
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads