Top Qs
Timeline
Chat
Perspective
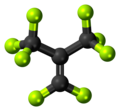
Perfluoroisobutene
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Perfluoroisobutene (PFIB) is the perfluorocarbon with the formula (CF3)2C=CF2. Classified as a perfluoroalkene, it is the fluorinated counterpart of the hydrocarbon isobutene. This colorless gas is notable for its high toxicity.[1]
Remove ads
Production and reactions
PFIB is one product of pyrolysis of polytetrafluoroethylene (PTFE).[2] Tetrafluoroethylene thermally dimerizes to octafluorocyclobutane, which above 600 °C degrades to hexafluoropropylene and PFIB.[1]
Perfluoroisobutene is highly reactive toward nucleophiles, e.g. methanol.[1] It also forms addition compounds with thiols, and it is this reactivity that may be related to its toxicity.[3] It hydrolyzes readily to give the relatively innocuous (CF3)2CHCO2H, which readily decarboxylates to give hexafluoropropane.[citation needed]
Oxidation of HFIB with potassium permanganate gives hexafluoroacetone.[4]
Remove ads
Safety
Perfluoroisobutene is highly toxic with an LCt = 880 mg⋅min⋅m−3 (mice).[3] It is a Schedule 2 substance of the Chemical Weapons Convention. Its toxicity is comparable to that of phosgene.[1]
References
External links
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads