Top Qs
Timeline
Chat
Perspective
Protactinium tetrafluoride
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Protactinium tetrafluoride is a binary inorganic compound of protactinium metal and fluorine with the chemical formula PaF4.[1]
Remove ads
Synthesis
Protactinium tetrafluoride can be obtained by fluorinating protactinium(IV) oxide with a hydrogen fluoride / hydrogen mixture at 600 °C:
- PaO2 + 4HF → PaF4 + 2H2O
The effect of hydrogen fluoride and hydrogen on protactinium(V) oxide:
- Pa2O5 + 8HF + H2 → 2PaF4 + 5H2O
Physical properties
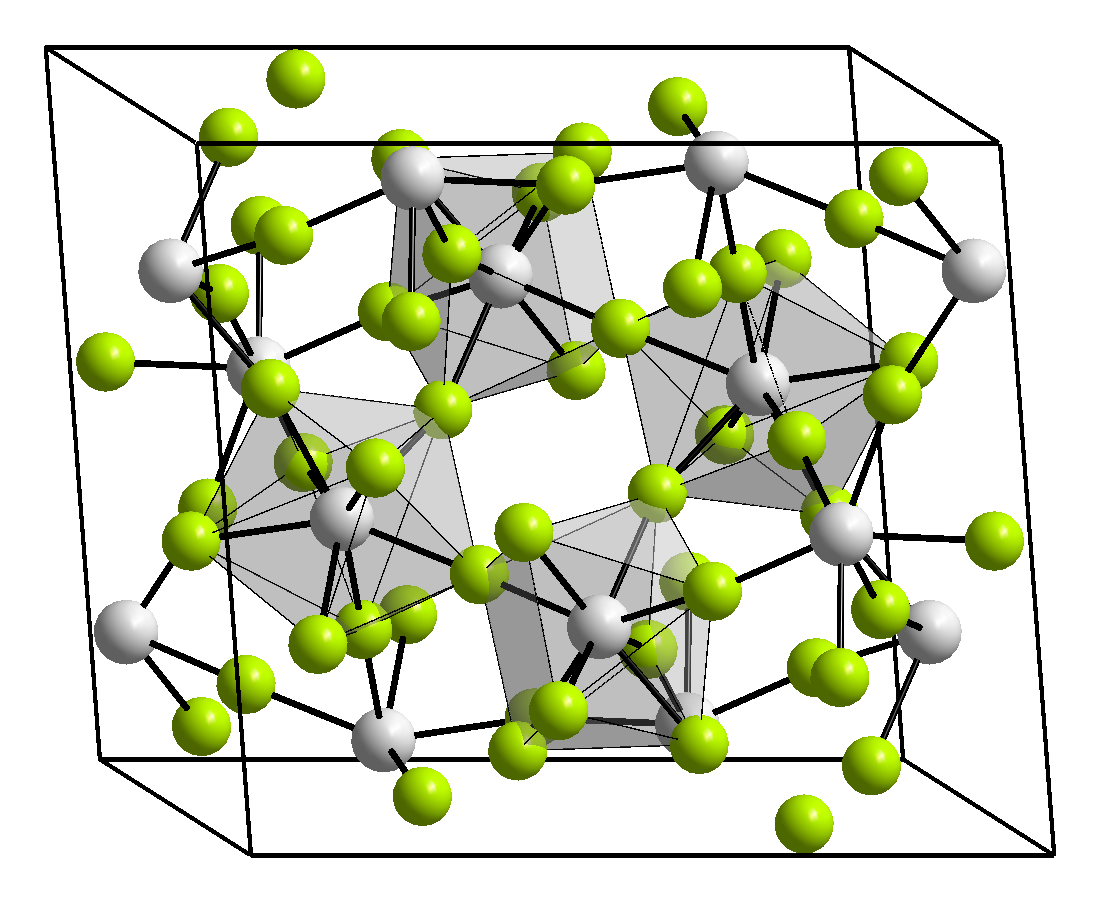
PaF4 forms dark brown, monoclinic, needlelike crystals of UF4 structure.[2][3] The cell parameters are: a = 1.27 nm, b = 1.07 nm, c = 0.842 nm, β = 126.3°.
The compound is soluble in aqueous ammonium fluoride solutions.[4]
Chemical properties
Protactinium tetrafluoride reacts with oxygen and fluorine:[2]
- 2PaF4 + F2 → 2PaF5
- 4PaF4 + O2 → 2Pa2OF8
The compound reacts with alkalis:
- PaF4 + 4NaOH → PaO2 + 4NaF + 2H2O
The metal is displaced from the salt by barium:
- PaF4 + 2Ba → Pa + 2BaF2
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads