Top Qs
Timeline
Chat
Perspective
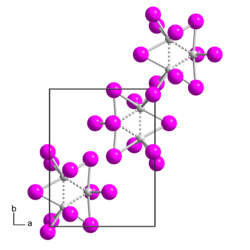
Rhenium(III) iodide
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Rhenium(III) iodide is a binary chemical compound of rhenium and iodide with the chemical formula ReI
3.[1][2]
Remove ads
Synthesis
Rhenium(III) iodide can be synthesized by the decomposition of rhenium(IV) iodide:[3][4]
- 2ReI4 → 2ReI3 + I2
Another way to make it is by introduction of ethanol into a mixture of perrhenic acid and hydroiodic acid.
- HReO4 + 3HI + 2C2H5OH → ReI3 + 4H2O + 2CH3CHO
Physical properties
Rhenium(III) iodide forms violet-black crystals. It is poorly soluble in water, acetone, ethanol, ether, and dilute acid solutions.
Chemical properties
When heated in vacuum up to 170 °C, the compound decomposes to rhenium(II) iodide, and at 380 °C — to rhenium(I) iodide:
- 2ReI3 → 2ReI2 + I2
- ReI3 → ReI + I2
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads