Top Qs
Timeline
Chat
Perspective
Tetrathiafulvalene
Organosulfuric compound with formula C6H4S4 From Wikipedia, the free encyclopedia
Remove ads
Tetrathiafulvalene (TTF) is an organosulfur compound with the formula H2C2S2C=CS2C2H2. It is the parent of many tetrathiafulvenes. Studies on these heterocyclic compound contributed to the development of molecular electronics, although no practical applications of TTF emerged. TTF is related to the hydrocarbon fulvalene (H4C4C=CC4H4) by replacement of four CH groups with sulfur atoms. Over 10,000 scientific publications discuss TTF and its derivatives.[2]
Remove ads
Preparation
The high level of interest in TTFs spawned many syntheses of TTF and its analogues.[3][2] Most preparations entail the coupling of cyclic C3S2 building blocks such as 1,3-dithiole-2-thion or the related 1,3-dithiole-2-ones. For TTF itself, the synthesis begins with the cyclic trithiocarbonate H2C2S2C=S (1,3-dithiole-2-thione), which is S-methylated and then reduced to give H2C2S2CH(SCH3) (1,3-dithiole-2-yl methyl thioether), which is treated as follows:[4]
Protonolysis of a thioether:
- H2C2S2CH(SCH3) + HBF4 → [H2C2S2CH]+BF−4 + CH3SH
Followed by deprotonation of the dithiolium cation with triethylamine:
- 2 [H2C2S2CH]+BF−4 + 2 N(CH2CH3)3 → H2C2S2C=CS2C2H2 + 2 [NH(CH2CH3)3]+BF−4
Remove ads
Redox properties
Bulk TTF itself has unremarkable electrical properties. Distinctive properties are, however, associated with salts of its oxidized derivatives, such as salts derived from TTF+.
The high electrical conductivity of TTF salts can be attributed to the following features of TTF:
- its planarity, which allows π-π stacking of its oxidized derivatives,
- its high symmetry, which promotes charge delocalization, thereby minimizing coulombic repulsions, and
- its ability to undergo oxidation at mild potentials to give a stable radical cation. Electrochemical measurements show that TTF can be oxidized twice reversibly:
Each dithiolylidene ring in TTF has 7π electrons: 2 for each sulfur atom, 1 for each sp2 carbon atom. Thus, oxidation converts each ring to an aromatic 6π-electron configuration, consequently leaving the central double bond essentially a single bond, as all π-electrons occupy ring orbitals.
Remove ads
History
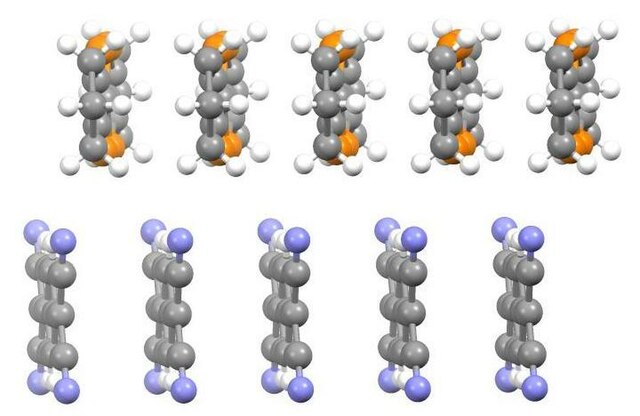
The salt [TTF+
]Cl−
was reported to be a semiconductor in 1972.[6] Subsequently, the charge-transfer salt [TTF]TCNQ was shown to be a narrow band gap semiconductor.[7] X-ray diffraction studies of [TTF][TCNQ] revealed stacks of partially oxidized TTF molecules adjacent to anionic stacks of TCNQ molecules. This "segregated stack" motif was unexpected and is responsible for the distinctive electrical properties, i.e. high and anisotropic electrical conductivity. Since these early discoveries, numerous analogues of TTF have been prepared. Well studied analogues include tetramethyltetrathiafulvalene (Me4TTF), tetramethylselenafulvalenes (TMTSFs), and bis(ethylenedithio)tetrathiafulvalene (BEDT-TTF, CAS [66946-48-3]).[8] Several tetramethyltetrathiafulvalene salts (called Fabre salts) are of some relevance as organic superconductors.
See also
References
Further reading
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads




