Top Qs
Timeline
Chat
Perspective
Technetium(VII) oxide
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
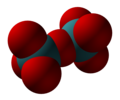
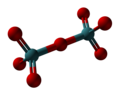
Technetium(VII) oxide is the chemical compound with the formula Tc2O7. This yellow volatile solid is a rare example of a molecular binary metal oxide, the other examples being RuO4, OsO4, and the unstable Mn2O7. It adopts a centrosymmetric corner-shared bi-tetrahedral structure in which the terminal and bridging Tc−O bonds are 167pm and 184 pm respectively and the Tc−O−Tc angle is 180°.[2]

Technetium(VII) oxide is prepared by the oxidation of technetium at 450–500 °C:[3]
- 4 Tc + 7 O2 → 2 Tc2O7
It is the anhydride of pertechnetic acid and the precursor to sodium pertechnetate:
- Tc2O7 + 2 H2O → 2 HTcO4
- Tc2O7 + 2 NaOH → 2 NaTcO4 + H2O
Remove ads
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads