Top Qs
Timeline
Chat
Perspective
Frémy's salt
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
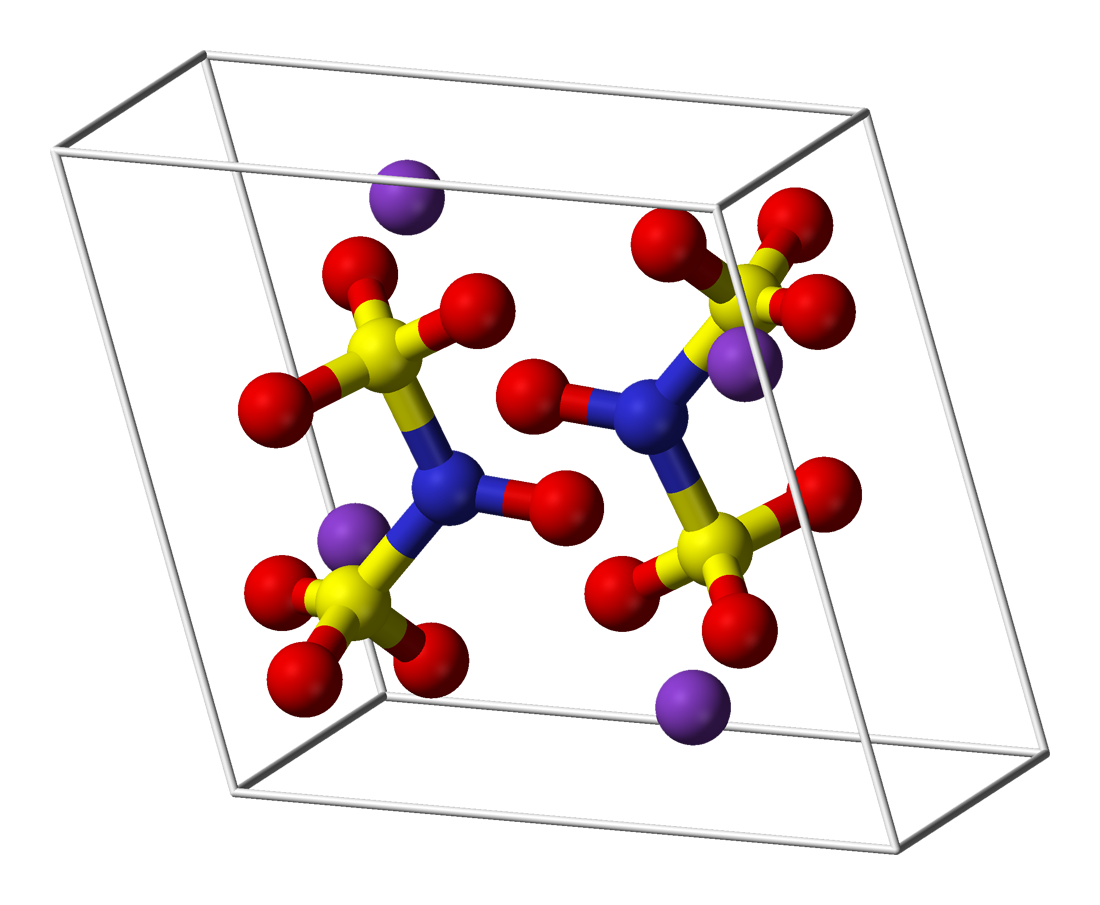
Frémy's salt is a chemical compound with the formula (K4[ON(SO3)2]2), sometimes written as (K2[NO(SO3)2]). It is a bright yellowish-brown solid, but its aqueous solutions are bright violet.[1][2] The related sodium salt, disodium nitrosodisulfonate (NDS, Na2ON(SO3)2, CAS 29554-37-8) is also referred to as Frémy's salt.[3]
Regardless of the cations, the salts are distinctive because aqueous solutions contain the radical [ON(SO3)2]2−.
Remove ads
Applications
Frémy's salt, being a long-lived free radical, is used as a standard in electron paramagnetic resonance (EPR) spectroscopy, e.g. for quantitation of radicals. Its intense EPR spectrum is dominated by three lines of equal intensity with a spacing of about 13 G (1.3 mT).[4][5][6]
The inorganic aminoxyl group is a persistent radical, akin to TEMPO.
It has been used in some oxidation reactions, such as for oxidation of some anilines and phenols[7][8][9][10][11] allowing polymerization and cross-linking of peptides and peptide-based hydrogels.[12][13]
It can also be used as a model for peroxyl radicals in studies that examine the antioxidant mechanism of action in a wide range of natural products.[14]
Remove ads
Preparation
Frémy's salt is prepared from hydroxylaminedisulfonic acid. Oxidation of the conjugate base gives the purple dianion:
- HON(SO3H)2 → [HON(SO3)2]2− + 2 H+
- 2 [HON(SO3)2]2− + PbO2 → 2 [ON(SO3)2]2− + PbO + H2O
The synthesis can be performed by combining nitrite and bisulfite to give the hydroxylaminedisulfonate. Oxidation is typically conducted at low-temperature, either chemically or by electrolysis.[3][2]
Other reactions:
- HNO2 + 2 HSO−
3 → HON(SO
3)2−
2 + H2O - 3 HON(SO
3)2−
2 + MnO−
4 + H+ → 3 ON(SO
3)2−
2 + MnO2 + 2 H2O - 2 ON(SO
3)2−
2 + 4 K+ → K4[ON(SO3)2]2
Remove ads
History
Frémy's salt was discovered in 1845 by Edmond Frémy (1814–1894).[15] Its use in organic synthesis was popularized by Hans Teuber, such that an oxidation using this salt is called the Teuber reaction.[9][10]
References
Further reading
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads