Top Qs
Timeline
Chat
Perspective
Thulium(III) chloride
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
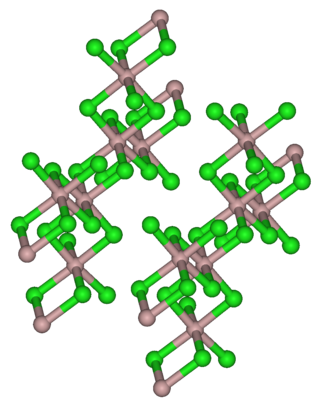
Thulium(III) chloride or thulium trichloride is as an inorganic salt composed of thulium and chlorine with the formula TmCl3. It forms yellow crystals. Thulium(III) chloride has the YCl3 (AlCl3) layer structure with octahedral thulium ions.[5] It has been used as a starting material for some exotic nanostructures prepared for NIR photocatalysis.[6][7]
Remove ads
Preparation
Thulium(III) chloride can be obtained by reacting thulium(III) oxide or thulium(III) carbonate and ammonium chloride:[8]
- Tm2O3 + 6 NH4Cl → 2 TmCl3 + 6 NH3 + 2 H2O
The hexahydrate of thulium(III) chloride can be obtained by adding thulium(III) oxide to concentrated hydrochloric acid.[1][8]
- 2 Tm + 6 HCl → 2 TmCl3 + 3 H2
Thulium(III) chloride can also be obtained by directly reacting thulium and chlorine:[9]
- 2 Tm + 3 Cl2 → 2 TmCl3
Remove ads
Properties
Thulium(III) chloride is a light yellow powder. Its hexahydrate is a light green hygroscopic solid.[6] Both are soluble in water.[10] Thulium(III) chloride has a monoclinic crystal structure with the space group C2/m (No. 12) corresponding to that of aluminum(III) chloride.[10][8]
Thulium(III) chloride reacts with strong bases to make thulium(III) oxide.
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads