Top Qs
Timeline
Chat
Perspective
Arsenic trioxide
Industrial chemical and medication (Arsenic-oxygen compound) From Wikipedia, the free encyclopedia
Remove ads
Arsenic trioxide is the inorganic compound with the formula As4O6.[3] As an industrial chemical, its major uses include the manufacture of wood preservatives, pesticides, and glass. For medical purposes, it is sold under the brand name Trisenox among others[4][5] when used as a medication to treat a type of cancer known as acute promyelocytic leukemia.[6] For this use it is given by injection into a vein.[6]
Arsenic trioxide was approved for medical use in the United States in 2000.[6] It is on the World Health Organization's List of Essential Medicines.[7] Approximately 50,000 tonnes were produced in 1991.[8] Due to its toxicity, a number of countries have regulations around its manufacture and sale.[9]
Remove ads
Uses
Summarize
Perspective
Arsenic trioxide is the dominant form of arsenic for commercial applications. Industrial uses include usage as a precursor to forestry products, in colorless glass production, and in electronics. Being the main compound of arsenic, the trioxide is the precursor to elemental arsenic, arsenic alloys, and arsenide semiconductors. Bulk arsenic-based compounds sodium arsenite and sodium cacodylate are derived from the trioxide.[8]
A variety of applications exploit arsenic's toxicity, including the use of the oxide as a wood preservative. Copper arsenates, such as chromated copper arsenate, are derived from arsenic trioxide. These compounds were once used on a large scale as wood preservatives in the U.S. and Malaysia, but are now banned in many parts of the world. This practice remains controversial.[8] When combined with copper(II) acetate, arsenic trioxide gives the vibrant green pigment known as Paris green, which finds some use as an insecticide.[10]
Medical
Historical
Despite the well known toxicity of arsenic, arsenic trioxide was used in traditional Chinese medicine, where it is known as pi-shuang (Chinese: 砒霜; pinyin: pīshuāng; lit. 'arsenic frost'). Some discredited patent medicines, e.g., Fowler's solution, contained derivatives of arsenic oxide.[11]
Modern
Arsenic trioxide is used to treat a type of cancer known as acute promyelocytic leukemia (APL).[6] It may be used both in cases that are unresponsive to other agents, such as all-trans retinoic acid (ATRA) or as part of the initial treatment of newly diagnosed cases.[6] This initial treatment may include combination therapy of arsenic trioxide with all-trans retinoic acid (ATRA).[12][13]
Remove ads
Production and occurrence
Summarize
Perspective

Arsenic trioxide can be generated via routine processing of arsenic compounds including the oxidation (combustion) of arsenic and arsenic-containing minerals in air. Illustrative is the roasting of orpiment, a typical arsenic sulfide ore.
- 2 As2S3 + 9 O2 → 2 As2O3 + 6 SO2
Smelting and related ore processing often generate arsenic trioxide, which poses a risk to the environment. For example, the Giant Mine in Canada processed substantial amounts of arsenopyrite-contaminated gold ores.
Most arsenic oxide is, however, obtained as a volatile by-product of the processing of other ores. For example, arsenopyrite, a common impurity in gold- and copper-containing ores, liberates arsenic trioxide upon heating in air. The processing of such minerals has led to numerous cases of poisonings,[14] and after the mine is closed, the leftover trioxide waste will present environmental hazard (as was the case with the Giant Mine, for example). Only in China are arsenic ores intentionally mined.[8]
In the laboratory, it is prepared by hydrolysis of arsenic trichloride:[15]
- 2 AsCl3 + 3 H2O → As2O3 + 6 HCl
As2O3 occurs naturally as two minerals, arsenolite (cubic) and claudetite (monoclinic). Both are relatively rare secondary minerals found in oxidation zones of As-rich ore deposits.
Remove ads
Reactions
Summarize
Perspective
Acid-base reactions
Arsenic trioxide is an amphoteric oxide, and its aqueous solutions are weakly acidic. Thus, it dissolves readily in alkaline solutions to give arsenites:[16]
- As2O3 + 6 NaOH → 2 Na3AsO3 + 3 H2O
Arsenic trioxide is less soluble in acids, although it will dissolve in hydrochloric acid.[17][page needed]
When treated with anhydrous HF and HCl, arsenic trioxide converts to the corresponding trihalide.[18] The tribromide and triiodide are made using concentrated hydrobromic acid and hydroiodic acid, respectively:[19]
- As2O3 + 6 HX → 2 AsX3 + 3 H2O (X = F, Cl, Br, I)
Redox reactions
Only with strong oxidizing agents such as ozone, hydrogen peroxide, and nitric acid does it yield arsenic pentoxide, As2O5 or its corresponding acid:[18]: 601
- 2 HNO3 + As2O3 + 2 H2O → 2 H3AsO4 + N2O3
In terms of its resistance to oxidation, arsenic trioxide differs from phosphorus trioxide, which readily combusts to phosphorus pentoxide.[clarification needed]
Reduction gives elemental arsenic or arsine (AsH3) depending on conditions:[18]: 593–594
- As2O3 + 6 Zn + 12 HNO3 → 2 AsH3 + 6 Zn(NO3)2 + 3 H2O
This reaction is used in the Marsh test.[20]
Precursor to organoarsenic compounds
Arsenic trioxide has played a special role as entry to organoarsenic chemistry. In the 18th century it was found that combining arsenic trioxide and four equivalents of potassium acetate (CH3CO2K) gives a product called "Cadet's fuming liquid", which is often considered the first organometallic compound. Cadet's fuming liquid is a derivative of cacodylic acid, ((CH3)2As)2O and cacodyl, ((CH3)2As)2.[21]
Arsenic trioxide reacts with phenyl magnesium bromide as described by the following idealized equation:[17]
- As2O3 + 4 C6H5MgBr → [(C6H5)2As]2O + 3 MgO + MgBr2
Metal derivatives
Like many other oxides, arsenic trioxide condenses with transition metal oxyanions to give polyoxometallates. Many such clusters have been characterized by X-ray crystallography.[22] It reacts with aqueous copper(II) acetate to give Cu(C2H3O2)2·3Cu(AsO2)2, known as Paris green.[23][24]
Structure
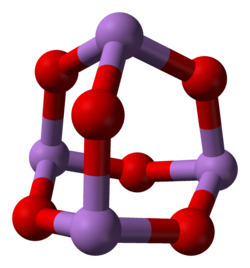
In the gas phase below 800 °C (1,470 °F), arsenic trioxide has the formula As4O6 and is isostructural with P4O6. Above 800 °C (1,470 °F) As4O6 dissociation into molecular As2O3, with the same structure as N2O3, becomes significant. Three crystalline forms (polymorphs) are known: a high temperature (over 110 °C (230 °F)) cubic form, containing molecular As4O6, and two related polymeric forms.[25][page needed] The polymers, which both crystallize as monoclinic crystals, feature sheets of pyramidal AsO3 units that share O atoms.[26][page needed] One of the polymeric forms (presumably I, as II was not known at the time) is apparently[27] the most stable form.
 |
 |
 |
arsenolite (cubic) | claudetite I (monoclinic) | claudetite II (monoclinic) |
The liquid state is agreed to be polymeric,[by whom?] and can form a glass; the liquid and glass have bonding of the same general type as the polymeric crystalline forms.[28]
Remove ads
Safety
As with other inorganic arsenic compounds, arsenic trioxide is toxic to living organisms. Arsenic trioxide is readily absorbed by the digestive system. Ingestion of as little as 100 mg can be fatal.[8]
Chronic arsenic poisoning is known as arsenicosis. This disorder affects workers in smelters, in populations whose drinking water contains high levels of arsenic (0.3–0.4 ppm), and in patients treated for long periods with arsenic-based pharmaceuticals. Long-term ingestion of arsenic trioxide either in drinking water or as a medical treatment can lead to skin cancer. Reproductive problems (high incidences of miscarriage, low birth weight, congenital deformations) have also been indicated in one study of women exposed to arsenic trioxide dust as employees or neighbours of a copper foundry.
In Austria, there lived the so-called "arsenic eaters of Styria", who ingested doses far beyond the lethal dose of arsenic trioxide without any apparent harm. Arsenic is thought to enable strenuous work at high altitudes, e.g. in the Alps.[29][30][31]
Remove ads
External links
- Landner L (2012). Chemicals in the Aquatic Environment: Advanced Hazard Assessment. Springer Science & Business Media. p. 259. ISBN 9783642613340. Archived from the original on 14 April 2023. Retrieved 18 March 2023.
- "Arsenic and Arsenic Compounds". Summaries & Evaluations. International Agency for Research on Cancer (IARC). February 1998.
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads