Top Qs
Timeline
Chat
Perspective
Tungsten dichloride dioxide
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Tungsten dichloride dioxide, or tungstyl chloride is the chemical compound with the formula WO2Cl2. It is a yellow solid. It is used as a precursor to other tungsten compounds. Like other tungsten halides, WO2Cl2 is sensitive to moisture, undergoing hydrolysis.
Remove ads
Preparation
WO2Cl2 is prepared by ligand redistribution reaction from tungsten trioxide and tungsten hexachloride:
- 2 WO3 + WCl6 → 3 WO2Cl2
Using a two-zone tube furnace, a vacuum-sealed tube containing these solids is heated to 350 °C. The yellow product sublimes to the cooler end of the reaction tube. No redox occurs in this process.[2] An alternative route highlights the oxophilicity of tungsten:[3]
- WCl6 + 2 ((CH3)3Si)2O → 3 WO2Cl2 + 4 (CH3)3SiCl
This reaction, like the preceding one, proceeds via the intermediacy of WOCl4.
Remove ads
Structure
Gaseous tungsten dichloride dioxide is a monomer.[4] Solid tungsten dichloride dioxide is a polymer consisting of distorted octahedral W centres. The polymer is characterized by two short W-O distances, typical for a multiple W-O bond, and two long W-O distances more typical of a single or dative W-O bond.[5]
Related oxyhalides
Tungsten forms a number of oxyhalides including WOCl4, WOCl3, WOCl2. The corresponding bromides (WOBr4, WOBr3, WOBr2) are also known as is WO2I2.[6]
Reactions
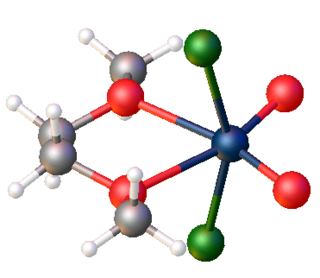
WO2Cl2 is a Lewis acid, forming soluble adducts of the type WO2Cl2L2, where L is a donor ligand such as bipyridine and dimethoxyethane. Such complexes often cannot be prepared by depolymerization of the inorganic solid, but are generated in situ from WOCl4.[7]
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads


