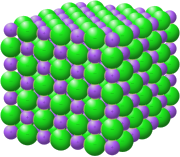
Ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions. It occurs mainly between metal and nonmetal atoms, where electrons are transferred from the metal atoms (which lose electrons to form positively charged ions called cations) to nonmetal atoms (which gain electrons to form negatively charged ions called anions). This transfer of electrons results in the formation of ionic compounds, characterized by a high melting point and conductivity in molten or dissolved states. In ionic bonding, the resulting ionic compound forms a crystal lattice structure, where the ions are arranged in an alternating pattern. The strength of the bonding is influenced by the charges of the ions and their relative sizes. The greater the charges, the stronger the attractive forces between the ions, leading to a higher melting point. Ionic bonds are generally stronger than covalent bonds when comparing similar ions. While ionic bonding is defined by the transfer of electrons, it is important to note that in practical scenarios, ionic compounds can exhibit some degree of covalent character due to the sharing of electron density between ions. The polarization effects, where the electron cloud of an anion is distorted by a small, highly charged cation, can introduce partial covalency in otherwise ionic bonding scenarios. Overall, ionic bonding occurs when the energy change of the formation of the compound is favorable, typically releasing energy during the formation of the crystal lattice.[1][2][3][4][5][6][7]
Showing results for Explain Ionic Bonding
Best Answer
AI GeneratedTop Questions
AI generatedMore questions