Selenium tetrachloride
chemical compound From Wikipedia, the free encyclopedia
Remove ads
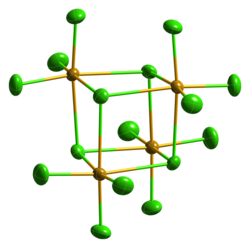
Selenium tetrachloride, also selenious chloride, selenous chloride, or selenium(IV) chloride, is a chemical compound made of selenium and chlorine. Its chemical formula is SeCl4. It contains selenium in its +4 oxidation state.
Remove ads
Properties
Selenium tetrachloride is a yellow or white solid. It evaporates easily. It reacts with water to make hydrochloric acid and selenous acid. It reacts with selenium dioxide to make selenium oxychloride, a mixture of selenium dioxide and selenium tetrachloride bonded together.
Preparation
It is made by heating a mixture of selenium and chlorine. The selenium tetrachloride escapes as a gas. This can be used to purify selenium. Impure selenium can be placed in the flask, reacted with chlorine. Only selenium tetrachloride escapes. This is reduced to selenium again, making pure selenium.
Uses
It is used to purify selenium. It is used to make other selenium compounds.
Related pages
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads