乙酸鋰
化合物 / 維基百科,自由的 encyclopedia
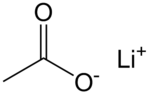
乙酸鋰是鋰的乙酸鹽,化學式為CH3COOLi。它在128 °C發生玻璃化轉變。[4]
Quick Facts 乙酸鋰, 識別 ...
| 乙酸鋰 | |
|---|---|
 | |
 | |
| 識別 | |
| CAS號 | 546-89-4(無水) 6108-17-4(二水) |
| PubChem | 11028 |
| ChemSpider | 10562 |
| SMILES |
|
| InChI |
|
| InChIKey | XIXADJRWDQXREU-REWHXWOFAX |
| EINECS | 208-914-3 |
| ChEBI | CHEBI:63045 |
| RTECS | AI545000 |
| KEGG | D08134 |
| MeSH | C488804 |
| 性質 | |
| 化學式 | C2H3LiO2 |
| 摩爾質量 | 65.99 g·mol−1 |
| 外觀 | 無色或白色晶體 |
| 密度 | 1.26 g/cm3 |
| 熔點 | 286 °C(559 K)(無水,熔化略有分解[1]) 28.3 °C(301.4 K)(二水)[2] |
| 溶解性(水) | 45.0 g/100 mL[3] |
| 磁化率 | −34.0·10−6 cm3/mol |
| 危險性 | |
| 致死量或濃度: | |
LD50(中位劑量)
|
500 mg/kg(小鼠,口服) |
| 若非註明,所有數據均出自標準狀態(25 ℃,100 kPa)下。 | |
Close