硫酸鋯是一種無機化合物,化學式為Zr(SO4)2,它可以以無水物、四水合物、五水合物和七水合物的形式存在。它們是白色可溶於水的固體。
| 硫酸鋯 | ||
|---|---|---|
|
| ||
| 英文名 | zirconium(IV) sulfate | |
| 別名 | 二硫酸鋯 硫酸鋯(IV) | |
| 識別 | ||
| CAS號 | 14644-61-2(無水) 7446-31-3(四水) | |
| PubChem | 26793(無水) 53249516(一水) | |
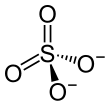
| SMILES |
| |
| RTECS | ZH9100000 | |
| 性質 | ||
| 化學式 | Zr(SO4)2(H2O)x ( x = 0, 4, 5, 7) | |
| 摩爾質量 | 285.35 g/mol(無水) 355.40(四水) g·mol⁻¹ | |
| 外觀 | 白色固體 | |
| 密度 | 3.22 g/cm3(無水) 2.85 g/cm3(四水) | |
| 溶解性(水) | 52.5 g(四水) | |
| 折光度n D |
1.646 | |
| 結構 | ||
| 晶體結構 | 正交晶系 | |
| 危險性 | ||
| 致死量或濃度: | ||
LD50(中位劑量)
|
3500 mg/kg(大鼠,口服)[1] | |
| 相關物質 | ||
| 其他陽離子 | 硫酸鈦 | |
| 若非註明,所有數據均出自標準狀態(25 ℃,100 kPa)下。 | ||
製備與結構
- ZrO2 + 2 H2SO4 + x H2O → Zr(SO4)2·xH2O
參考文獻
Wikiwand in your browser!
Seamless Wikipedia browsing. On steroids.
Every time you click a link to Wikipedia, Wiktionary or Wikiquote in your browser's search results, it will show the modern Wikiwand interface.
Wikiwand extension is a five stars, simple, with minimum permission required to keep your browsing private, safe and transparent.