Loading AI tools
Cancerous tumor originating in the stomach lining From Wikipedia, the free encyclopedia
Stomach cancer, also known as gastric cancer, is a cancer that develops from the lining of the stomach.[10] Most cases of stomach cancers are gastric carcinomas, which can be divided into a number of subtypes, including gastric adenocarcinomas.[2] Lymphomas and mesenchymal tumors may also develop in the stomach.[2] Early symptoms may include heartburn, upper abdominal pain, nausea, and loss of appetite.[1] Later signs and symptoms may include weight loss, yellowing of the skin and whites of the eyes, vomiting, difficulty swallowing, and blood in the stool, among others.[1] The cancer may spread from the stomach to other parts of the body, particularly the liver, lungs, bones, lining of the abdomen, and lymph nodes.[11]
| Stomach cancer | |
|---|---|
| Other names | Gastric cancer |
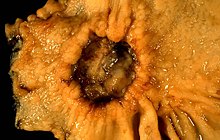 | |
| A stomach ulcer that was diagnosed as cancer on biopsy | |
| Specialty | Gastroenterology Oncology |
| Symptoms | Early: Later: |
| Usual onset | Over years[2] |
| Types | Gastric carcinomas, lymphoma, mesenchymal tumor[2] |
| Causes | Helicobacter pylori, genetics[2][3] |
| Risk factors | Smoking, dietary factors such as pickled vegetables, obesity[2][4] |
| Diagnostic method | Biopsy done during endoscopy[1] |
| Prevention | Mediterranean diet, not smoking[2][5] |
| Treatment | Surgery, chemotherapy, radiation therapy, targeted therapy[1] |
| Prognosis | Five-year survival rate: < 10% (advanced cases),[6] 32% (US),[7] 71% (Japan)[8] |
| Frequency | 968,350 (2022)[9] |
| Deaths | 659,853 (2022)[9] |
The bacterium Helicobacter pylori accounts for more than 60% of cases of stomach cancer[2][3][12] Certain strains of H. pylori have greater risks than others.[2] Smoking, dietary factors such as pickled vegetables and obesity are other risk factors.[2][4] About 10% of cases run in families, and between 1% and 3% of cases are due to genetic syndromes inherited such as hereditary diffuse gastric cancer.[2] Most of the time, stomach cancer develops in stages over years.[2] Diagnosis is usually by biopsy done during endoscopy.[1] This is followed by medical imaging to determine if the disease has spread to other parts of the body.[1] Japan and South Korea, two countries that have high rates of the disease, screen for stomach cancer.[2]
A Mediterranean diet lowers the risk of stomach cancer, as does not smoking.[2][5] Tentative evidence indicates that treating H. pylori decreases the future risk.[2][5] If stomach cancer is treated early, it can be cured.[2] Treatments may include some combination of surgery, chemotherapy, radiation therapy, and targeted therapy.[1][13] For certain subtypes of gastric cancer, cancer immunotherapy is an option as well.[14] If treated late, palliative care may be advised.[2] Some types of lymphoma can be cured by eliminating H. pylori.[15] Outcomes are often poor, with a less than 10% five-year survival rate in the Western world for advanced cases.[6] This is largely because most people with the condition present with advanced disease.[6] In the United States, five-year survival is 31.5%,[7] while in South Korea it is over 65% and Japan over 70%, partly due to screening efforts.[2][8]
Globally, stomach cancer is the fifth-leading type of cancer and the third-leading cause of death from cancer, making up 7% of cases and 9% of deaths.[16] In 2018, it newly occurred in 1.03 million people and caused 783,000 deaths.[17] Before the 1930s, it was a leading cause of cancer deaths in the Western world, however rates have sharply declined among younger generations in the West, while they remain high for people living in East Asia.[18][19][20] The decline in the West is believed to be due to the decline of salted and pickled food consumption, as a result of the development of refrigeration as a method of preserving food.[21] Stomach cancer occurs most commonly in East Asia, followed by Eastern Europe.[2] It occurs twice as often in males as in females.[2]


Stomach cancer is often either asymptomatic (producing no noticeable symptoms) or it may cause only nonspecific symptoms (which may also be present in other related or unrelated disorders) in its early stages. By the time symptoms are recognized, the cancer has often reached an advanced stage (see below) and may have metastasized (spread to other, perhaps distant, parts of the body), which is one of the main reasons for its relatively poor prognosis.[22] Stomach cancer can cause the following signs and symptoms: Unexplained nausea, vomiting, diarrhoea and constipation. Patients also can experience unexplained weight loss.[23]
Early cancers may be associated with indigestion or a burning sensation (heartburn). However, fewer than one in every 50 people referred for endoscopy due to indigestion has cancer.[24] Abdominal discomfort and loss of appetite can occur.[25][26]
Gastric cancers that have enlarged and invaded normal tissue can cause weakness, fatigue, bloating of the stomach after meals, abdominal pain in the upper abdomen, nausea and occasional vomiting. Further enlargement may cause weight loss or bleeding with vomiting blood or having blood in the stool, the latter apparent as black discolouration (melena) and sometimes leading to anemia. Dysphagia suggests a tumour in the cardia or extension of the gastric tumour into the esophagus.[citation needed]
These can be symptoms of other problems such as a stomach virus, gastric ulcer, or tropical sprue.[citation needed]
Gastric cancer can occur as a result of many factors.[27] It occurs twice as commonly in males as females. Estrogen may protect women against the development of this form of cancer.[28][29]
Helicobacter pylori infection is an essential risk factor in 65–80% of gastric cancers, but only 2% of people with H. pylori infections develop stomach cancer.[4][30] The mechanism by which H. pylori induces stomach cancer potentially involves chronic inflammation, the action of H. pylori virulence factors such as CagA,[31] or an interaction between H. pylori infection and germline pathogenic variants in homologous-recombination genes.[32] It was estimated that Epstein–Barr virus is responsible for 84,000 cases per year.[33] AIDS is also associated with elevated risk.[4]
Smoking increases the risk of developing gastric cancer significantly, from 40% increased risk for current smokers to 82% increase for heavy smokers. Gastric cancers due to smoking mostly occur in the upper part of the stomach near the esophagus.[34][35][36]
Some studies show increased risk with alcohol consumption as well.[4][37]
Dietary factors are not proven causes, and the association between stomach cancer and various foods and beverages is weak.[38] Some foods including fried foods,[39] smoked foods, salt and salt-rich foods, meat,[40] processed meat,[40] red meat,[40] pickled vegetables, and brackens[41] are associated with a higher risk of stomach cancer.[4][42]
Fresh fruit and vegetable intake,[43] citrus fruit intake,[43] and antioxidant intake are associated with a lower risk of stomach cancer.[4][34] A Mediterranean diet is associated with lower rates of stomach cancer,[44] as is regular aspirin use.[4]
Obesity is a physical risk factor that has been found to increase the risk of gastric adenocarcinoma by contributing to the development of gastroesophageal reflux disease (GERD).[45] The exact mechanism by which obesity causes GERD is not completely known. Studies hypothesize that increased dietary fat leading to increased pressure on the stomach and the lower esophageal sphincter, due to excess adipose tissue, could play a role, yet no statistically significant data have been collected.[46] However, the risk of gastric cardia adenocarcinoma, with GERD present, has been found to increase more than two times for an obese person.[45] There is a correlation between iodine deficiency and gastric cancer.[47]
About 10% of cases run in families, and between 1 and 3% of cases are due to genetic syndromes inherited such as hereditary diffuse gastric cancer.[2]
A genetic risk factor for gastric cancer is a genetic defect of the CDH1 gene known as hereditary diffuse gastric cancer (HDGC). The CDH1 gene, which codes for E-cadherin, lies on the 16th chromosome.[48] When the gene experiences a particular mutation, gastric cancer develops through a mechanism that is not fully understood.[48][49] This mutation is considered autosomal dominant, meaning that half of a carrier's children will likely experience the same mutation.[48] Diagnosis of hereditary diffuse gastric cancer usually takes place when at least two cases involving a family member, such as a parent or grandparent, are diagnosed, with at least one diagnosed before the age of 50.[48] The diagnosis can also be made if at least three cases occur in the family, in which case age is not considered.[48]
The International Cancer Genome Consortium is leading efforts to identify genomic changes involved in stomach cancer.[50][51] A very small percentage of diffuse-type gastric cancers (see Histopathology below) arise from an inherited abnormal CDH1 gene. Genetic testing and treatment options are available for families at risk.[52]
To find the cause of symptoms, the doctor asks about the patient's medical history, does a physical examination, and may order laboratory studies.[53] The patient may also have one or all of these exams:
In 2013, Chinese and Israeli scientists reported a successful pilot study of a breathalyzer-style breath test intended to diagnose stomach cancer by analyzing exhaled chemicals without the need for an intrusive endoscopy.[55][56] A larger-scale clinical trial of this technology was completed in 2014.[57][58]
Abnormal tissue seen in a gastroscope examination is biopsied by the surgeon or gastroenterologist. This tissue is then sent to a pathologist for histological examination under a microscope to check for the presence of cancerous cells. A biopsy, with subsequent histological analysis, is the only sure way to confirm the presence of cancer cells.[37]
Various gastroscopic modalities have been developed to increase yield of detected mucosa with a dye that accentuates the cell structure and can identify areas of dysplasia. Endocytoscopy involves ultra-high magnification to visualise cellular structure to better determine areas of dysplasia. Other gastroscopic modalities such as optical coherence tomography are being tested investigationally for similar applications.[59]
A number of cutaneous conditions are associated with gastric cancer. A condition of darkened hyperplasia of the skin, frequently of the axilla and groin, known as acanthosis nigricans, is associated with intra-abdominal cancers such as gastric cancer. Other cutaneous manifestations of gastric cancer include "tripe palms" (a similar darkening hyperplasia of the skin of the palms) and the Leser-Trelat sign, which is the rapid development of skin lesions known as seborrheic keratoses.[60]
Various blood tests may be done, including a complete blood count to check for anaemia, and a fecal occult blood test to check for blood in the stool.[61]


If cancer cells are found in the tissue sample, the next step is to stage, or find out the extent of the disease. Various tests determine whether the cancer has spread, and if so, what parts of the body are affected. Because stomach cancer can spread to the liver, pancreas, and other organs near the stomach, as well as to the lungs, the doctor may order a CT scan, a PET scan,[67] an endoscopic ultrasound exam, or other tests to check these areas. Blood tests for tumor markers, such as carcinoembryonic antigen and carbohydrate antigen may be ordered, as their levels correlate to extent of metastasis, especially to the liver, and the cure rate.[citation needed]
Staging may not be complete until after surgery. The surgeon removes nearby lymph nodes and possibly samples of tissue from other areas in the abdomen for examination by a pathologist.[citation needed]
The clinical stages of stomach cancer are:[68][69]

The TNM staging system is also used.[70]
In a study of open-access endoscopy in Scotland, patients were diagnosed 7% in stage I, 17% in stage II, and 28% in stage III.[71] A Minnesota population was diagnosed 10% in stage I, 13% in stage II, and 18% in stage III.[72] However, in a high-risk population in the Valdivia Province of southern Chile, only 5% of patients were diagnosed in the first two stages and 10% in stage III.[73]
Getting rid of H. pylori in those who are infected decreases the risk of stomach cancer.[74] A 2014 meta-analysis of observational studies found that a diet high in fruits, mushrooms, garlic, soybeans, and green onions was associated with a lower risk of stomach cancer in the Korean population.[75] Low doses of vitamins, especially from a healthy diet, decrease the risk of stomach cancer.[76] A previous review of antioxidant supplementation did not find supporting evidence and possibly worse outcomes.[77][78] Modern technology is used to promote early diagnosis, e.g. based on serum markers.[79]
Cancer of the stomach is difficult to cure unless it is found at an early stage (before it has begun to spread). Unfortunately, because early stomach cancer causes few symptoms, the disease is usually advanced when the diagnosis is made.[80]
Treatment for stomach cancer may include surgery,[81] chemotherapy,[13] or radiation therapy.[82] New treatment approaches such as immunotherapy or gene therapy and improved ways of using current methods are being studied in clinical trials.[83]

Surgery remains the only curative therapy for stomach cancer.[6] A 2016 Cochrane review found low-quality evidence of no difference in short-term mortality between laparoscopic and open gastrectomy (removal of stomach), and that benefits or harms of laparoscopic gastrectomy cannot be ruled out.[84] Post-operatively, up to 70% of people undergoing total gastrectomy develop complications such as dumping syndrome and reflux esophagitis.[85] Construction of a "pouch", which serves as a "stomach substitute", reduced the incidence of dumping syndrome and reflux esophagitis by 73% and 63% respectively, and led to improvements in quality-of-life, nutritional outcomes, and body mass index.[85] Proximal gastrectomy (PG) can be considered a viable alternative for upper third early gastric cancer (EGC)[86] Of the different surgical techniques, endoscopic mucosal resection (EMR) is a treatment for early gastric cancer (tumor only involves the mucosa) that was pioneered in Japan and is available in the United States at some centers.[6] In EMR, the tumor, together with the inner lining of stomach (mucosa), is removed from the wall of the stomach using an electrical wire loop through the endoscope. The advantage is that it is a much smaller operation than removing the stomach.[6] Endoscopic submucosal dissection is a similar technique pioneered in Japan, used to resect a large area of mucosa in one piece.[6] If the pathologic examination of the resected specimen shows incomplete resection or deep invasion by tumor, the patient would need a formal stomach resection.[6]
Those with metastatic disease at the time of presentation may receive palliative surgery, and while it remains controversial, due to the possibility of complications from the surgery itself and because it may delay chemotherapy, the data so far are mostly positive, with improved survival rates being seen in those treated with this approach.[6][87]
The use of chemotherapy to treat stomach cancer has no firmly established standard of care.[13] Unfortunately, stomach cancer has not been particularly sensitive to these drugs, and chemotherapy, if used, has usually served to palliatively reduce the size of the tumor, relieve symptoms of the disease, and increase survival time.[13] Some drugs used in stomach cancer treatment have included: fluorouracil or its analog capecitabine, BCNU (carmustine), methyl-CCNU (semustine) and doxorubicin (Adriamycin), as well as mitomycin C, and more recently cisplatin and taxotere, often using drugs in various combinations.[13] The relative benefits of these different drugs, alone and in combination, are unclear.[13][88] Clinical researchers are exploring the benefits of giving chemotherapy before surgery to shrink the tumor, or as adjuvant therapy after surgery to destroy remaining cancer cells.[6]
Recently[when?], treatment with human epidermal growth factor receptor 2 (HER2) inhibitor, trastuzumab, has been demonstrated to increase overall survival in inoperable locally advanced or metastatic gastric carcinoma over-expressing the HER2/neu gene.[6] In particular, HER2 is overexpressed in 13–22% of patients with gastric cancer.[83][89] Of note, HER2 overexpression in gastric neoplasia is heterogeneous and comprises a minority of tumor cells (less than 10% of gastric cancers overexpress HER2 in more than 5% of tumor cells). Hence, this heterogeneous expression should be taken into account for HER2 testing, particularly in small samples such as biopsies, requiring the evaluation of more than one bioptic sample.[89]
A recent clinical study reported promising results for a combination therapy using Nivolumab and Anlotinib in the treatment of advanced gastric adenocarcinoma (GAC) and esophageal squamous cell carcinoma (ESCC), that improve the immune response against cancer while simultaneously slowing tumor progression.[90]. The research, conducted by Zhongshan Hospital, Fudan University, and BGI Genomics, was published in Nature Communications in October 2024. The study evaluated the efficacy of combining Nivolumab, an immunotherapy that enhances the immune system's ability to attack cancer cells, with anlotinib hydrochloride, a drug that inhibits tumor angiogenesis by blocking signals essential for the growth of new blood vessels[91].
Radiation therapy (also called radiotherapy) may be used to treat stomach cancer, often as an adjuvant to chemotherapy and/or surgery.[6]
MALT lymphomas are often completely resolved after the underlying H. pylori infection is treated.[15] This results in remission in about 80% of cases.[15]
The prognosis of stomach cancer is generally poor, because the tumor has often metastasized by the time of discovery, and most people with the condition are elderly (median age is between 70 and 75 years) at presentation.[92] The average life expectancy after being diagnosed is around 24 months, and the five-year survival rate for stomach cancer is less than 10%.[6]
Almost 300 genes are related to outcomes in stomach cancer, with both unfavorable genes where high expression is related to poor survival and favorable genes where high expression is associated with longer survival times.[93][94] Examples of poor prognosis genes include ITGAV, DUSP1 and P2RX7.[95]

In 2018, stomach cancer was the fifth most frequently diagnosed cancer worldwide, representing 5.7% of all cancer cases, and the third leading cause of death from cancers, being responsible for 8.2% of all cancer deaths.[96] Among men, 683 754 cases were diagnosed, accounting for 7.2% of all cancer cases, and among women, stomach cancer was diagnosed in 349 947 cases, accounting for 4.1% of all cancer cases.[96]
In 2012, stomach cancer was the fifth most-common cancer with 952,000 cases diagnosed.[16] It is more common both in men and in developing countries.[97][98] In 2012, it represented 8.5% of cancer cases in men, making it the fourth most-common cancer in men.[99] Also in 2012, the number of deaths was 700,000, having decreased slightly from 774,000 in 1990, making it the third-leading cause of cancer-related death (after lung cancer and liver cancer).[100][101]
Less than 5% of stomach cancers occur in people under 40 years of age, with 81.1% of that 5% in the age-group of 30 to 39 and 18.9% in the age-group of 20 to 29.[102]
In 2014, stomach cancer resulted in 0.61% of deaths (13,303 cases) in the United States.[103] In China, stomach cancer accounted for 3.56% of all deaths (324,439 cases).[104][unreliable source?] The highest rate of stomach cancer was in Mongolia, at 28 cases per 100,000 people.[105][unreliable source?]
In the United Kingdom, stomach cancer is the 15th most-common cancer (around 7,100 people were diagnosed with stomach cancer in 2011), and it is the 10th most-common cause of cancer-related deaths (around 4,800 people died in 2012).[106]
Incidence and mortality rates of gastric cancer vary greatly in Africa. The GLOBOCAN system is currently the most widely used method to compare these rates between countries, but African incidence and mortality rates are seen to differ among countries, possibly due to the lack of universal access to a registry system for all countries.[107] Variation as drastic as estimated rates from 0.3/100000 in Botswana to 20.3/100000 in Mali have been observed.[107] In Uganda, the incidence of gastric cancer has increased from the 1960s measurement of 0.8/100000 to 5.6/100000.[107] Gastric cancer, though present, is relatively low when compared to countries with high incidence like Japan and China. One suspected cause of the variation within Africa and between other countries is due to different strains of the H. pylori bacteria. The trend commonly seen is that H. pylori infection increases the risk for gastric cancer, but this is not the case in Africa, giving this phenomenon the name the "African enigma".[108] Although this bacterial species is found in Africa, evidence has supported that different strains with mutations in the bacterial genotype may contribute to the difference in cancer development between African countries and others outside the continent.[108] Increasing access to health care and treatment measures have been commonly associated with the rising incidence, though, particularly in Uganda.[107]
The stomach is a muscular organ of the gastrointestinal tract that holds food and begins the digestive process by secreting gastric juice. The most common cancers of the stomach are adenocarcinomas, but other histological types have been reported. Signs vary, but may include vomiting (especially if blood is present), weight loss, anemia, and lack of appetite. Bowel movements may be dark and tarry in nature. To determine whether cancer is present in the stomach, special X-rays and/or abdominal ultrasounds may be performed. Gastroscopy, a test using an endoscope to examine the stomach, is a useful diagnostic tool that can also take samples of the suspected mass for histopathological analysis to confirm or rule out cancer. The most definitive method of cancer diagnosis is through open surgical biopsy.[109] Most stomach tumors are malignant with evidence of spread to lymph nodes or liver, making treatment difficult. Except for lymphoma, surgery is the most frequent treatment option for stomach cancers but it is associated with significant risks.[citation needed]
A carcinogenic interaction was demonstrated between bile acids and Helicobacter pylori in a mouse model of gastric cancer.[110][111]
Seamless Wikipedia browsing. On steroids.
Every time you click a link to Wikipedia, Wiktionary or Wikiquote in your browser's search results, it will show the modern Wikiwand interface.
Wikiwand extension is a five stars, simple, with minimum permission required to keep your browsing private, safe and transparent.