Chăng-kō̤ Mìng-dĕ̤ng-ngṳ̄ Háng-cê gì bēng-buōng. / 參考閩東語漢字其版本。
快速預覽 gái-huóng, miàng-cê·hù-hô̤·sê̤ṳ-só ...
Seaborgi 106Sg|
|
| gái-huóng |
|---|
| miàng-cê·hù-hô̤·sê̤ṳ-só |
Seaborgi (seaborgium)·Sg·106 |
|---|
| nguòng-só lôi-biék |
guó-dô gĭng-sṳ̆k |
|---|
| cŭk·ciŭ-gĭ·kṳ̆ |
6·7·d |
|---|
| nguòng-cṳ̄-liông |
[269] |
|---|
| diêng-cṳ̄ bà̤-buó |
[Rn] 5f14 6d4 7s2
2, 8, 18, 32, 32, 12, 2
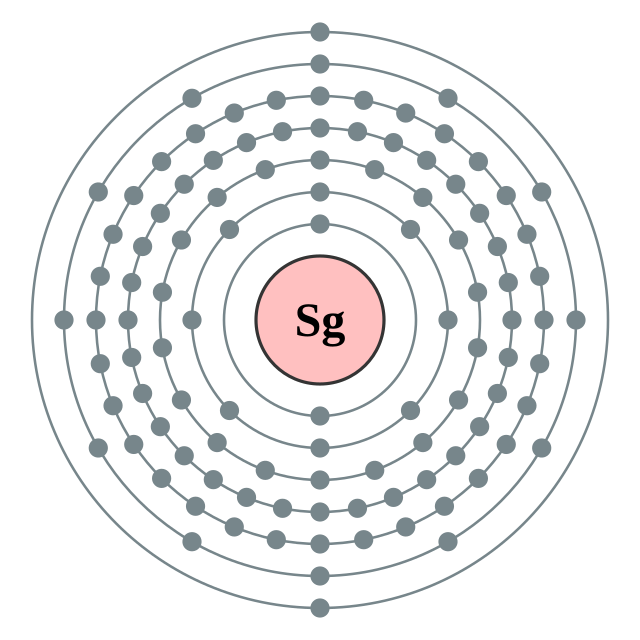 Seaborgi gì diêng cṳ̄ cèng (2, 8, 18, 32, 32, 12, 2) Seaborgi gì diêng cṳ̄ cèng (2, 8, 18, 32, 32, 12, 2) |
|---|
| Lĭk-sṳ̄ |
|---|
| huák-hiêng |
Lawrence Berkeley National Laboratory(1974 nièng) |
|---|
| ŭk-lī séng-cék |
|---|
| ŭk-tái |
gó-tā̤(predicted)[1] |
|---|
| mĭk-dô̤ |
(ciék-gê̤ṳng sék-ŭng)
35.0 g·cm−3 |
|---|
cĭng-ké-ák
|
| nguòng-cṳ̄ séng-cék |
|---|
| iōng-huá-tái |
6, (5), (4), (3), 0
((parenthesized oxidation states are predictions)) |
|---|
| diêng-liê-nèng |
dâ̤ 1: {{{1st ionization energy}}} kJ·mol−1
dâ̤ 2: {{{2nd ionization energy}}} kJ·mol−1
dâ̤ 3: {{{3rd ionization energy}}} kJ·mol−1
|
|---|
| nguòng-cṳ̄ buáng-géng |
132 pm |
|---|
| gê̤ṳng-gá buáng-géng |
143 pm
((estimated)[2]) |
|---|
| gì-tă |
|---|
| cĭng-tā̤ giék-gáiu |
body-centered cubic
(predicted)[1] |
|---|
| CAS hô̤ |
54038-81-2 |
|---|
| có̤i ūng-diâng gì dùng-ôi-só |
|---|
|
| dùng-ôi-só |
hŭng-dô |
buáng-sŏi-gĭ |
huŏng-sék |
nèng-liông(MeV) |
sāng-ŭk |
|---|
| 271Sg |
syn |
1.9 min |
67% α |
8.54 |
267Rf |
| 33% SF |
|
|
| 269Sg |
syn |
3.1模板:Su min |
α |
8.50(6) |
265Rf |
| 267Sg |
syn |
1.4 min |
17% α |
8.20 |
263Rf |
| 83% SF |
|
|
| 265mSg |
syn |
16.2 s |
α |
8.70 |
261mRf |
| 265Sg |
syn |
8.9 s |
α |
8.90, 8.84, 8.76 |
261Rf |
|
關閉
Seaborgium sê siŏh cṳ̄ng gĭng-sṳ̆k, iâ sê siŏh cṳ̄ng huá-hŏk nguòng-só. Nguòng-cṳ̄ sê̤ṳ-só sê 106, huá-hŏk hù-hô̤ sê Sg.

