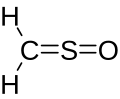Top Qs
Timeline
Chat
Perspective
Sulfine
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Sulfinylmethane or sulfine is an organic compound with molecular formula H2CSO. It is the simplest sulfine. Sulfines are chemical compounds with the general structure XY=SO.[1] IUPAC considers the term 'sulfine' obsolete,[2] preferring instead thiocarbonyl S-oxide; despite this, the use of the term sulfine still predominates in the chemical literature.
Remove ads
Substituted sulfines
The parent sulfine H2CSO is very labile, whereas substituted derivatives are more conveniently isolated.
One route is a variant of ketene synthesis, in which a sulfinyl halide reacts with a hindered base. For example, syn-propanethial-S-oxide, responsible for eye-watering effects of cutting onions, is produced so from allicin.[3]
Another route is oxidation, as with thiobenzophenone from diphenylsulfine:[4]
- (C6H5)2C=S + [O] → (C6H5)2C=S=O

Remove ads
See also
- Sulfene - related functional group with the formula H2C=SO2
- Ethenone
- Heterocumulene
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads