Top Qs
Timeline
Chat
Perspective
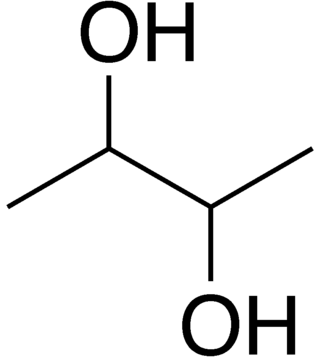
2,3-Butanediol
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
2,3-Butanediol is the organic compound with the formula (CH3CHOH)2. It is classified as a vic-diol (glycol). It exists as three stereoisomers, a chiral pair and the meso isomer. All are colorless liquids. Applications include precursors to various plastics and pesticides.
Remove ads
Isomerism
Of the three stereoisomers, two are enantiomers (levo- and dextro-2,3-butanediol) and one is a meso compound.[1][2] The enantiomeric pair have (2R, 3R) and (2S, 3S) configurations at carbons 2 and 3, while the meso compound has configuration (2R, 3S) or, equivalently, (2S, 3R).
Industrial production and uses
2,3-Butanediol is prepared by hydrolysis of 2,3-epoxybutane:[3]
- (CH3CH)2O + H2O → CH3(CHOH)2CH3
The isomer distribution depends on the stereochemistry of the epoxide.
The meso isomer is used to combine with naphthalene-1,5-diisocyanate. The resulting polyurethane is called "Vulkollan".[3]
Biological production
The (2R,3R)-stereoisomer of 2,3-butanediol is produced by a variety of microorganisms in a process known as butanediol fermentation.[4] It is found naturally in cocoa butter, in the roots of Ruta graveolens, sweet corn, and in rotten mussels. It is used in the resolution of carbonyl compounds in gas chromatography.[5]
During World War II research was done towards producing 2,3-butanediol by fermentation in order to produce 1,3-butadiene, the monomer of the polybutadiene used in a leading type of synthetic rubber.[6] It can be derived from the fermentation of sugarcane molasses.[7]
Fermentative production of 2,3-butanediol from carbohydrates involves a network of biochemical reactions that can be manipulated to maximize production.[8]
2,3-butanediol has been proposed as a rocket fuel that could be created on Mars by means of cyanobacteria and E. coli, shipped from Earth, working on resources available at the surface of Mars.[9]
2,3-Butanediol has been detected, in peppers, grape wine, anatidaes.
Reactions
2,3-Butanediol undergo dehydration to form butanone (methyl ethyl ketone):[10]
- (CH3CHOH)2 → CH3C(O)CH2CH3 + H2O
It can also undergo deoxydehydration to form butene:[11]
- (CH3CHOH)2 + 2 H2 → C4H8 + 2 H2O
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads