Top Qs
Timeline
Chat
Perspective
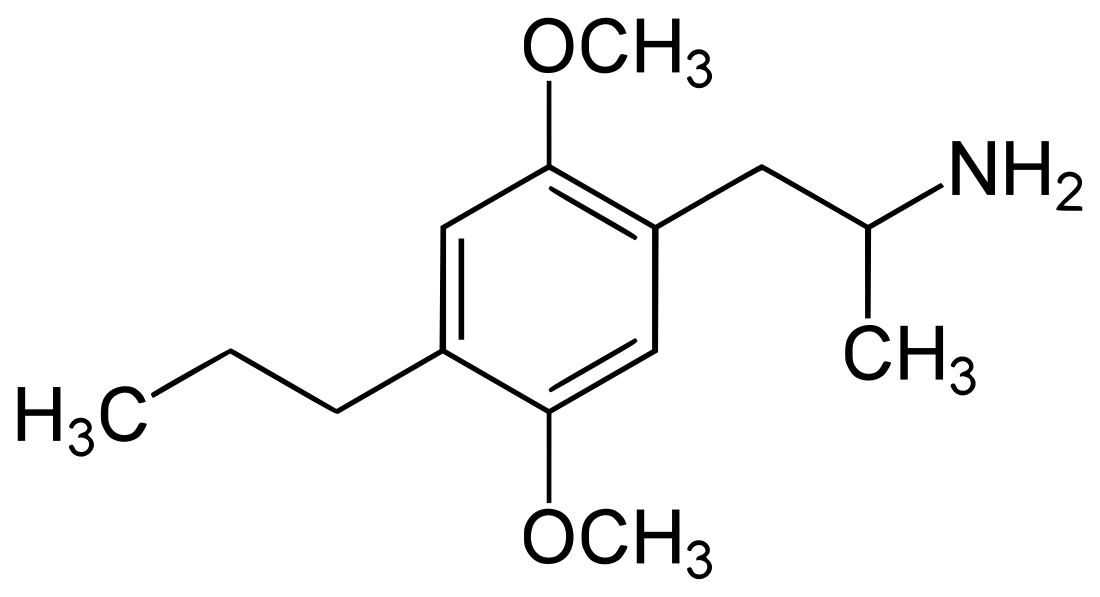
2,5-Dimethoxy-4-propylamphetamine
Psychedelic drug From Wikipedia, the free encyclopedia
Remove ads
2,5-Dimethoxy-4-propylamphetamine (DOPR) is a psychedelic drug of the phenethylamine, amphetamine, and DOx families related to DOM.[1][2] It is the derivative of DOM in which the methyl group at the 4 position has been replaced with a propyl group.[1] The drug is taken orally.[1]
The drug acts as a serotonin receptor agonist, including of the serotonin 5-HT2A receptor.[2][3] It produces psychedelic-like effects in animals.[2][3]
DOPR was first described in the literature by Alexander Shulgin in 1970.[4] Subsequently, it was described in greater detail by Shulgin in his 1991 book PiHKAL (Phenethylamines I Have Known and Loved).[1]
Remove ads
Use and effects
In his book PiHKAL (Phenethylamines I Have Known and Loved), Alexander Shulgin lists DOPR's dose as 2.5 to 5 mg orally and its duration as 20 to 30 hours.[1] It is said to have a very slow onset.[1] The effects of DOPR have been reported to include closed-eye imagery, visuals, thinking changes, and insomnia and sleep disruption, among others.[1] In one of the reports, it was described as a "heavy duty psychedelic", including strong and unignorable visuals.[1]
Remove ads
Interactions
Pharmacology
Summarize
Perspective
Pharmacodynamics
DOPR acts as an agonist of the serotonin 5-HT2 receptors, including of the serotonin 5-HT2A, 5-HT2B, and 5-HT2C receptors.[2][5][6][3][7] It has very weak affinity for the serotonin 5-HT1 receptor.[8] The drug has also been assessed at other receptors.[2]
It produces the head-twitch response (HTR), a behavioral proxy of psychedelic effects, in rodents.[2][3] It is slightly more potent but slightly less efficacious than DOM in producing the head-twitch response.[2] As with many other psychedelics, DOPR shows an inverted U-shaped dose–response curve in terms of the HTR, increasing it at lower doses and having diminished effectiveness at higher doses.[2][3]
DOPR showed no significant effects on locomotor activity in rodents at the assessed doses, but showed a trend towards hyperlocomotion at the highest dose.[3] In a subsequent study however, it produced hyperlocomotion at lower doses and hypolocomotion at higher doses.[2] The drug has shown pro-motivational effects in rodents at sub-hallucinogenic doses or so-called "microdoses".[5][6] DOPR's close analogue DOET has also been clinically studied at sub-hallucinogenic doses as a "psychic energizer".[9][10][11][12][13][14]
At higher doses, DOPR produces hypothermia in rodents.[2]
Pharmacokinetics
DOPR crosses the blood–brain barrier in rodents.[2] The drug showed the highest brain/plasma ratio among DOM homologues in rodents, whereas 2,5-dimethoxyamphetamine (2,5-DMA) showed the lowest.[2] This was involved in potency differences between the drugs.[2]
Chemistry
Synthesis
The chemical synthesis of DOPR has been described.[1]
Analogues
The alternative structural isomer DOIP, with a 4-isopropyl substitution, is also known but is around ten times weaker than DOPR, with an active dose of some 20–30 mg (as compared to 2–5 mg for DOPR).[1]
History
DOPR was first described in the literature by Alexander Shulgin in 1970.[4] Subsequently, it was described in greater detail by Shulgin in his 1991 book PiHKAL (Phenethylamines I Have Known and Loved).[1]
See also
References
External links
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads