Top Qs
Timeline
Chat
Perspective
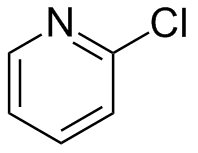
2-Chloropyridine
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
2-Chloropyridine is an aryl chloride with the formula C5H4ClN. It is a colorless liquid that is mainly used to generate fungicides and insecticides in industry. It also serves to generate antihistamines and antiarrythymics for pharmaceutical purposes.[2] It is one of three isomers of chloropyridine.
Remove ads
Preparation
2-Chloropyridine is produced by direct reaction of pyridine with chlorine. The initially formed 2-chloropyridine reacts further to give 2,6-dichloropyridine.[2]
Alternatively, 2-chloropyridines can be conveniently synthesized in high yields from pyridine-N-oxides.[3]
2-Chloropyridine was originally prepared by the chlorination of 2-hydroxypyridine with phosphoryl chloride.[4]
Main reactions and applications
2-Chloropyridine reacts with nucleophiles to generate pyridine derivatives substituted at the second and fourth carbons on the heterocycle. Therefore, many reactions using 2-chloropyridine generate mixtures of products which require further workup to isolate the desired isomer.[2]
Some commercial products include pyrithione, pyripropoxyfen, chlorphenamine, and disopyramide. In these conversions, chloride is displaced.[2] Pyrithione, the conjugate base of 2-mercaptopyridine-N-oxide, is a fungicide found in some shampoos. Oxidation 2-chloropyridine gives 2-chloropyridine-N-oxide.[5] The antihistamine pheniramine may be generated via the reaction of phenylacetonitrile with 2-chloropyridine in the presence of a base.[6]
Remove ads
Environmental properties
Although pyridine is an excellent source of carbon, nitrogen, and energy for certain microorganisms, introduction of a halogen moiety significantly retards degradation of the pyridine ring. With the exception of 4-chloropyridine, each of the mono- and di-substituted chloropyridines were found to be relatively resistant to microbiological degradation in soil or liquid media.[7] Estimated time for complete degradation was > 30 days. 2-Chloropyridine exhibits extensive volatilization losses from water, less so when present in soil.[8]
Toxicity
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads