Top Qs
Timeline
Chat
Perspective
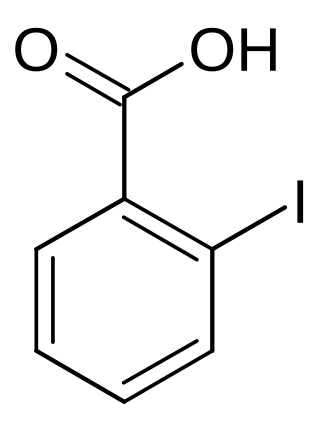
2-Iodobenzoic acid
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
2-Iodobenzoic acid, or o-iodobenzoic acid, is an isomer of iodobenzoic acid.[1] The synthesis of 2-iodobenzoic acid via the diazotization of anthranilic acid is commonly performed in university organic chemistry labs. One of its most common uses is as a precursor for the preparation of IBX and Dess–Martin periodinane, both used as mild oxidants.
Remove ads
Synthesis
2-Iodobenzoic acid can be synthesized by a Sandmeyer reaction: the diazotization of anthranilic acid followed by a reaction with iodide.

See also
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads