Top Qs
Timeline
Chat
Perspective
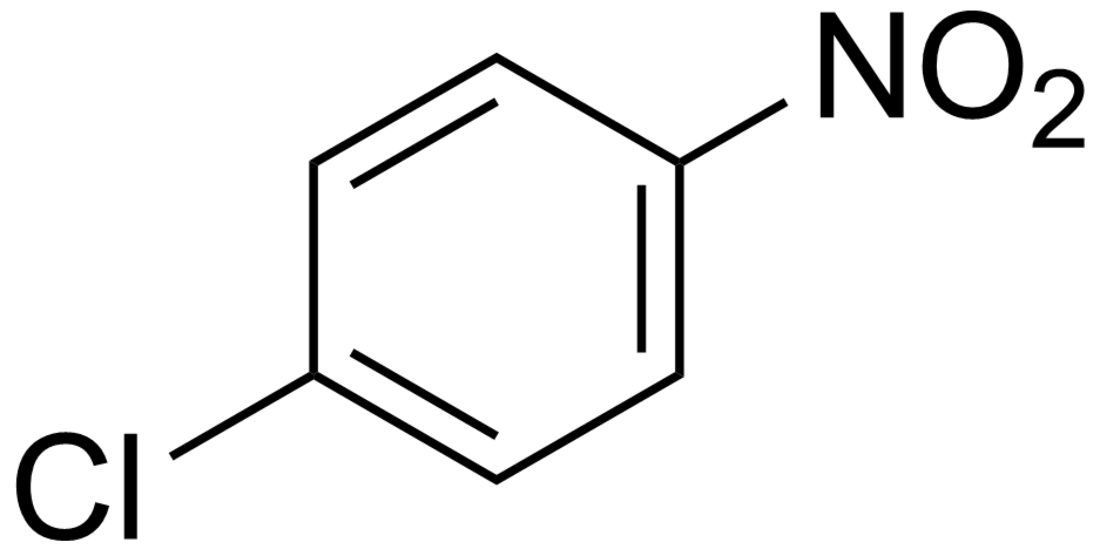
4-Nitrochlorobenzene
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
4-Nitrochlorobenzene is the organic compound with the formula ClC6H4NO2. It is a pale yellow solid. 4-Nitrochlorobenzene is a common intermediate in the production of a number of industrially useful compounds, including antioxidants commonly found in rubber. Other isomers with the formula ClC6H4NO2 include 2-nitrochlorobenzene and 3-nitrochlorobenzene.
Remove ads
Preparation and reactions
4-Nitrochlorobenzene is prepared industrially by nitration of chlorobenzene:
- ClC6H5 + HNO3 → ClC6H4NO2 + H2O
This reaction affords both the 2- and the 4-nitro derivatives, in about a 1:2 ratio. These isomers are separated by a combination of crystallization and distillation.[2] 4-Nitrochlorobenzene was originally prepared by the nitration of 4-bromochlorobenzene by Holleman and coworkers.[3]
The chloride substituent in 4-nitrochlorobenzene is more labile than in chlorobenzene. For example, it is readily displaced by sulfide nucleophiles, leading the way to 4-nitrothiophenol.[4] In another example, 4-nitrochlorobenzene is a favored substrate for cross-coupling reactions.[5]
Remove ads
Applications
4-Nitrochlorobenzene is an intermediate in the preparation of a variety of derivatives. Nitration gives 2,4-dinitrochlorobenzene, and 3,4-dichloronitrobenzene. Reduction with iron metal gives 4-chloroaniline. The electron-withdrawing nature of the appended nitro-group makes the benzene ring especially susceptible to nucleophilic aromatic substitution, unlike related chlorobenzene. Thus, the strong nucleophiles hydroxide, methoxide, fluoride, and amide displace chloride to give respectively 4-nitrophenol, 4-nitroanisole, 4-fluoronitrobenzene, and 4-nitroaniline.[2][6]
Another use of 4-nitrochlorobenzene is its condensation with aniline to produce 4-nitrodiphenylamine. Reductive alkylation of the nitro group affords secondary aryl amines, which are useful antioxidants for rubber.
Remove ads
Safety
The U.S. National Institute for Occupational Safety and Health considers 4-nitrochlorobenzene as a potential occupational carcinogen.[7] The Occupational Safety and Health Administration set a permissible exposure limit of 1 mg/m3 The American Conference of Governmental Industrial Hygienists recommends an airborne exposure limit of 0.64 mg/m3 over a time-weighted average of eight hours.[8][9]
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads