Top Qs
Timeline
Chat
Perspective
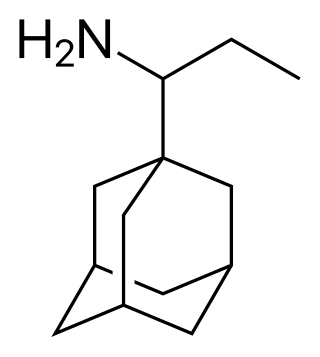
Adapromine
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Adapromine is an antiviral drug of the adamantane group related to amantadine (1-aminoadamantane), rimantadine (1-(1-aminoethyl)adamantane), and memantine (1-amino-3,5-dimethyladamantane) that is marketed in Russia for the treatment and prevention of influenza.[1][2][3][4] It is an alkyl analogue of rimantadine and is similar to rimantadine in its antiviral activity but possesses a broader spectrum of action, being effective against influenza viruses of both type A and B.[1][2][5] Strains of type A influenza virus with resistance to adapromine and rimantadine and the related drug deitiforine were encountered in Mongolia and the Soviet Union in the 1980s.[6][7]
Electroencephalography (EEG) studies of animals suggest that adapromine and related adamantanes including amantadine, bromantane (1-amino-2-bromophenyladamantane), and memantine have psychostimulant-like and possibly antidepressant-like effects, and that these effects may be mediated via catecholaminergic processes.[8][9][10][11] These psychostimulant effects differ qualitatively from those of conventional psychostimulants like amphetamine however, and the adamantane derivatives have been described contrarily as "adaptogens" and as "actoprotectors".[12]
In 2004, it was discovered that amantadine and memantine bind to and act as agonists of the σ1 receptor (Ki = 7.44 μM and 2.60 μM, respectively) and that activation of the σ1 receptor is involved in the dopaminergic effects of amantadine at therapeutically relevant concentrations.[13] These findings might also extend to the other adamantanes such as adapromine, rimantadine, and bromantane and could explain the psychostimulant-like effects of this family of compounds.[13]
Remove ads
Synthesis
The first synthesis of adapromine was disclosed in patents by DuPont published in 1967.[14]
1-Adamantanecarboxylic acid, as its acid chloride, is treated with a cadmium-modified Grignard reagent, which gives the ketone (6). Oxime formation with hydroxylamine, followed by reduction using lithium aluminium hydride yields adapromine.[14][15]
See also
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads