Top Qs
Timeline
Chat
Perspective
Aldo-keto reductase
Protein family From Wikipedia, the free encyclopedia
Remove ads
The aldo-keto reductase family is a family of proteins that are subdivided into 16 categories; these include a number of related monomeric NADPH-dependent oxidoreductases, such as aldehyde reductase, aldose reductase, prostaglandin F synthase, xylose reductase, rho crystallin, and many others.[1]
Remove ads
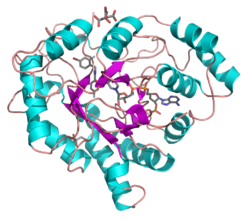
Structure
All possess a similar structure, with a beta-alpha-beta fold characteristic of nucleotide binding proteins.[2] The fold comprises a parallel beta-8/alpha-8-barrel, which contains a novel NADP-binding motif. The binding site is located in a large, deep, elliptical pocket in the C-terminal end of the beta sheet, the substrate being bound in an extended conformation. The hydrophobic nature of the pocket favours aromatic and apolar substrates over highly polar ones.[3]
Binding of the NADPH coenzyme causes a massive conformational change, reorienting a loop, effectively locking the coenzyme in place. This binding is more similar to FAD- than to NAD(P)-binding oxidoreductases.[4]
Remove ads
Examples
Some proteins of this family contain a potassium channel beta chain regulatory domain; these are reported to have oxidoreductase activity.[5]
See also
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads