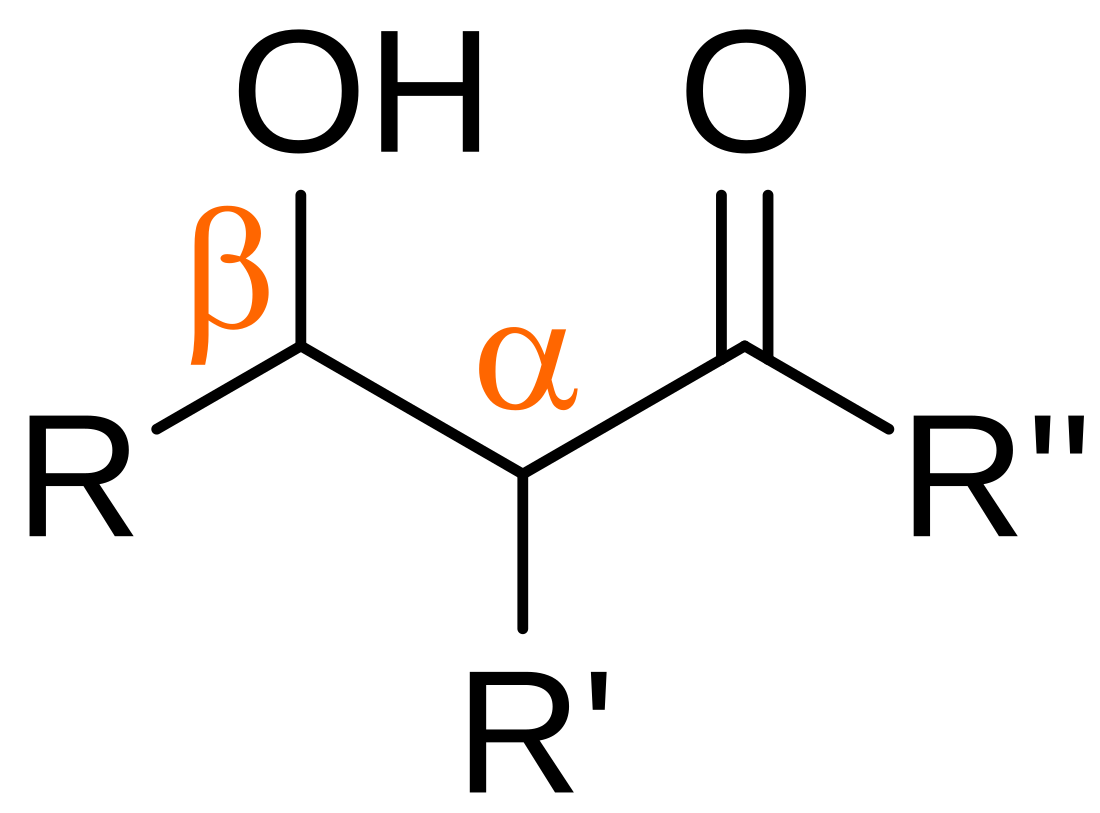Top Qs
Timeline
Chat
Perspective
Aldol
Organic compound of the form R–CH(OH)–CHR–C(=O)–R From Wikipedia, the free encyclopedia
Remove ads
In organic chemistry, an aldol is a structure consisting of a hydroxy group (-OH) two carbons away from either an aldehyde or a ketone. The name combines the suffix 'ol' from the alcohol and the prefix depending on the carbonyl group, either 'ald' for an aldehyde, or 'ket' for a ketone, in which case it referred to as a 'ketol'. An aldol may also use the term β-hydroxy aldehyde (or β-hydroxy ketone for a ketol). The term "aldol" may refer to 3-hydroxybutanal.[1][2]

Aldols are the product of a carbon-carbon bond-formation reaction, giving them wide applicability as a precursor for a variety of other compounds.
Remove ads
Synthesis and reactions

Aldols are usually synthesized from an aldol addition reaction using two aldehydes or an aldehyde and a ketone for a ketol.[1] These reactions may also be done intramolecularly to form 5 or 6 member rings or for stereoselective syntheses in the active area of asymmetric synthesis.
Aldols may also undergo a condensation reaction in which the hydroxy group is replaced by a pi bond. The final structure is a reactive α,β-unsaturated carbonyl compound, which can also be used in a variety of other reactions:
- RC(O)CH2CH(OH)R' → RC(O)CH=CHR' + H2O
Remove ads
Applications
Aldols synthesized from two aldehydes are usually unstable, often producing secondary compounds such as diols, unsaturated aldehydes, or alcohols.[1] Hydroxypivaldehyde is a rare example of a distillable aldol.[3] The aldol 3-hydroxybutanal is a precursor to quinaldine, which is a precursor to the dye quinoline Yellow SS.[1]
Aldols are also used as intermediates in the synthesis of polyketide natural products and drugs such as Oseltamivir and Epothilone.[4][5][6][7]
Remove ads
See also
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads