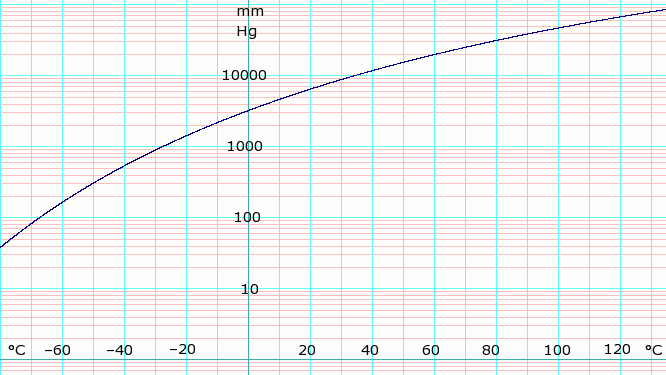
P in mm Hg 1 10 40 100
400 760 1520 3800 7600 15600 30400 45600
T in °C −109.1(s) −91.9(s) −79.2(s) −68.4 −45.4 −33.6 −18.7 4.7 25.7 50.1 78.9 98.3
Table data (above) obtained from CRC Handbook of Chemistry and Physics 44th ed. The (s) notation indicates equilibrium temperature of vapor over solid. Otherwise temperature is equilibrium of vapor over liquid.
log10 of anhydrous ammonia vapor pressure. Uses formula shown below.
Vapor-pressure formula for ammonia:[ 4]
log10 P = A – B / (T − C ), where P is pressure in kPa , and T is temperature in kelvins ;
A = 6.67956, B = 1002.711, C = 25.215 for T = 190 K through 333 K.More information Vapor over anhydrous ammonia, Vapor over aqueous ammonia solution ...
Vapor over anhydrous ammonia[ 5]
Temp.
Pressure
ρ of liquidρ of vaporΔ vap H
−78 °C 5.90 kPa
−75 °C 7.93 kPa
0.73094 g/cm3
7.8241× 10−5 g/cm3
−70 °C 10.92 kPa
0.72527 g/cm3
1.1141× 10−4 g/cm3
−65 °C 15.61 kPa
0.71953 g/cm3
1.5552× 10−4 g/cm3
−60 °C 21.90 kPa
0.71378 g/cm3
2.1321× 10−4 g/cm3
−55 °C 30.16 kPa
0.70791 g/cm3
2.8596× 10−4 g/cm3
−50 °C 40.87 kPa
0.70200 g/cm3
3.8158× 10−4 g/cm3
1417 J/g
−45 °C 54.54 kPa
0.69604 g/cm3
4.9940× 10−4 g/cm3
1404 J/g
−40 °C 71.77 kPa
0.68999 g/cm3
6.4508× 10−4 g/cm3
1390 J/g
−35 °C 93.19 kPa
0.68385 g/cm3
8.2318× 10−4 g/cm3
1375 J/g
−30 °C 119.6 kPa
0.67764 g/cm3
1.0386× 10−3 g/cm3
1361 J/g
−25 °C 151.6 kPa
0.67137 g/cm3
1.2969× 10−3 g/cm3
1345 J/g
−20 °C 190.2 kPa
0.66503 g/cm3
1.6039× 10−3 g/cm3
1330 J/g
−15 °C 236.3 kPa
0.65854 g/cm3
1.9659× 10−3 g/cm3
1314 J/g
−10 °C 290.8 kPa
0.65198 g/cm3
2.3874× 10−3 g/cm3
1297 J/g
−5 °C 354.8 kPa
0.64533 g/cm3
2.8827× 10−3 g/cm3
1280 J/g
0 °C429.4 kPa
0.63857 g/cm3
3.4528× 10−3 g/cm3
1263 J/g
5 °C515.7 kPa
0.63167 g/cm3
4.1086× 10−3 g/cm3
1245 J/g
10 °C614.9 kPa
0.62469 g/cm3
4.8593× 10−3 g/cm3
1226 J/g
15 °C728.3 kPa
0.61755 g/cm3
5.7153× 10−3 g/cm3
1207 J/g
20 °C857.1 kPa
0.61028 g/cm3
6.6876× 10−3 g/cm3
1187 J/g
25 °C1003 kPa
0.60285 g/cm3
7.7882× 10−3 g/cm3
1167 J/g
30 °C1166 kPa
0.59524 g/cm3
9.0310× 10−3 g/cm3
1146 J/g
35 °C1350 kPa
0.58816 g/cm3
1.0431× 10−2 g/cm3
1124 J/g
40 °C1554 kPa
0.57948 g/cm3
1.2006× 10−2 g/cm3
1101 J/g
45 °C1781 kPa
0.57130 g/cm3
1.3775× 10−2 g/cm3
1083 J/g
50 °C2032 kPa
0.56287 g/cm3
1.5761× 10−2 g/cm3
1052 J/g
55 °C2310 kPa
0.55420 g/cm3
60 °C2613 kPa
0.54523 g/cm3
2.05× 10−2 g/cm3
65 °C2947 kPa
0.53596 g/cm3
70 °C3312 kPa
0.52632 g/cm3
2.65× 10−2 g/cm3
75 °C3711 kPa
0.51626 g/cm3
80 °C4144 kPa
0.50571 g/cm3
3.41× 10−2 g/cm3
85 °C4614 kPa
0.49463 g/cm3
90 °C5123 kPa
0.48290 g/cm3
4.39× 10−2 g/cm3
95 °C5672 kPa
0.47041 g/cm3
100 °C 6264 kPa
0.45693 g/cm3
5.68× 10−2 g/cm3
Temp.
Pressure
ρ of liquidρ of vaporΔ vap H
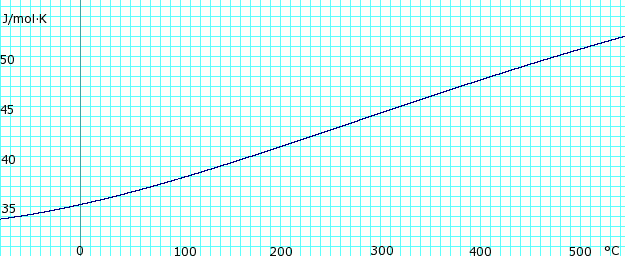
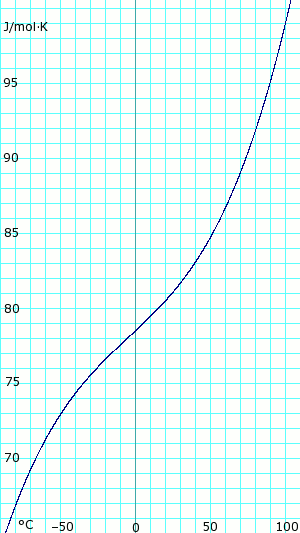
The table above gives properties of the vapor–liquid equilibrium of anhydrous ammonia at various temperatures. The second column is vapor pressure in kPa . The third column is the density of the liquid phase. The fourth column is the density of the vapor. The fifth column is the heat of vaporization needed to convert one gram of liquid to vapor.
Freezing curve of ammonia-water system. Three eutectic points I. II. and III. are shown. Left of the I. point the frozen component is ice. Right of the III. point the frozen component is ammonia.[ 6]
Vapor over aqueous ammonia solution[ 7]
Temp.
%wt NH3
Partial pressure3
Partial pressure2 O
0 °C 4.72 1.52 kPa
0.68 kPa
9.15 3.31 kPa 0.71 kPa
14.73 6.84 kPa 0.55 kPa
19.62 11.0 kPa 0.40 kPa
22.90 14.9 kPa 0.37 kPa
10 °C 4.16 2.20 kPa
1.21 kPa
8.26 4.96 kPa 1.17 kPa
12.32 8.56 kPa 1.01 kPa
15.88 12.68 kPa
0.93 kPa
20.54 19.89 kPa
0.83 kPa
21.83 22.64 kPa
0.73 kPa
19.9 °C 4.18 3.65 kPa
2.19 kPa
6.50 6.11 kPa 2.15 kPa
6.55 6.13 kPa 2.13 kPa
7.72 7.49 kPa 2.08 kPa
10.15 10.75 kPa
2.01 kPa
10.75 11.51 kPa
1.96 kPa
16.64 22.14 kPa
1.72 kPa
19.40 28.74 kPa
1.64 kPa
23.37 40.32 kPa
1.37 kPa
30.09 °C 3.93 5.49 kPa
4.15 kPa
7.43 11.51 kPa 3.89 kPa
9.75 16.00 kPa 3.80 kPa
12.77 23.33 kPa
3.55 kPa
17.76 38.69 kPa
3.31 kPa
17.84 38.81 kPa
3.24 kPa
21.47 53.94 kPa
2.95 kPa
40 °C 3.79 8.15 kPa
7.13 kPa
7.36 17.73 kPa 6.76 kPa
11.06 29.13 kPa
6.55 kPa
15.55 47.14 kPa
5.52 kPa
17.33 57.02 kPa
20.85 76.81 kPa
5.04 kPa
50 °C 3.29 10.54 kPa
11.95 kPa
5.90 20.17 kPa 11.61 kPa
8.91 32.88 kPa 11.07 kPa
11.57 45.56 kPa
10.75 kPa
14.15 60.18 kPa
10.27 kPa
14.94 64.94 kPa
10.03 kPa
60 °C 3.86 18.25 kPa
19.21 kPa
5.77 28.78 kPa
7.78 40.05 kPa 18.47 kPa
9.37 50.09 kPa 18.07 kPa
9.37 63.43 kPa 17.39 kPa
Temp.
%wt NH3
Partial Pressure3
Partial Pressure2 O
Close