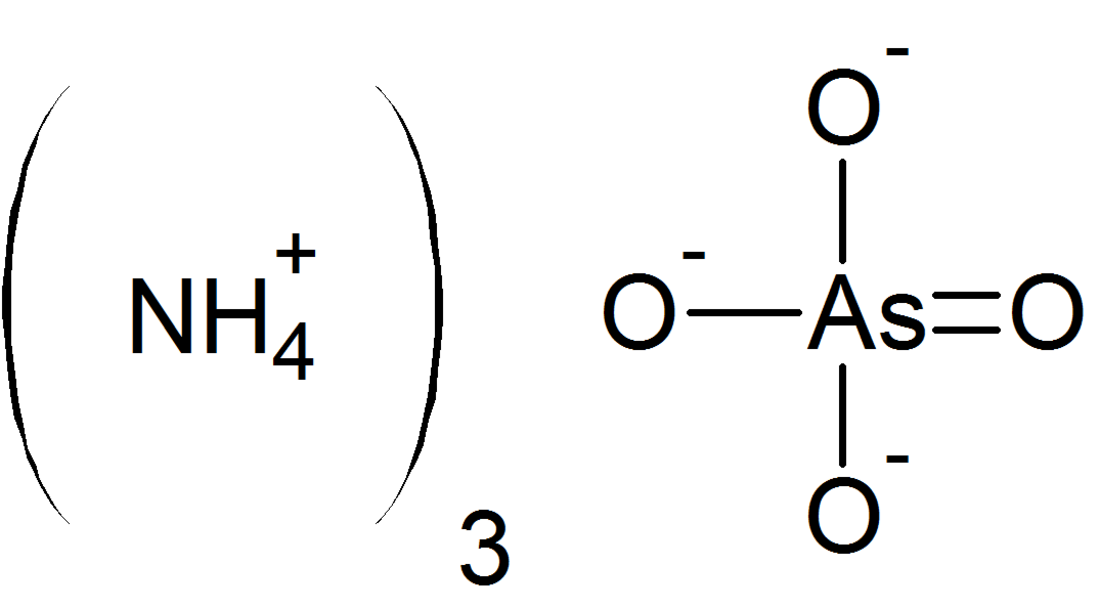Top Qs
Timeline
Chat
Perspective
Ammonium arsenate
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Ammonium arsenate is an inorganic compound with the formula ((NH4)3AsO4, typically encountered as a trihydrate, (NH4)3AsO4·3H2O. It is a colorless, water-soluble crystalline solid that decomposes upon heating, releasing ammonia and forming arsenic-containing residues.
Classified as an IARC Group 1 carcinogen, it is highly toxic and poses significant health and environmental risks.[1][2]
Historically used in pesticides and analytical chemistry, its applications are now limited due to toxicity concerns. Ammonium arsenate occurs rarely in nature and is primarily synthesized for research or industrial purposes. Its chemistry, environmental behavior, and analytical detection are of interest in toxicology, environmental chemistry, and biogeochemistry.[3]
It is prepared by treating a concentrated solution of arsenic acid with ammonia, resulting in precipitation of colorless crystals of the trihydrate.[4] Upon heating, it releases ammonia.
Acid salts are also known, including diammonium arsenate and ammonium dihydrogen arsenate.
Remove ads
Chemistry and structure
Summarize
Perspective
Ammonium arsenate consists of ammonium cations (NH4+) and the arsenate anion (AsO43-), where arsenic is in the +5 oxidation state, tetrahedrally coordinated to four oxygen atoms. The trihydrate form, (NH4)3AsO4·3H2O, is the most common, featuring a crystal lattice stabilized by hydrogen bonding between water molecules, ammonium ions, and arsenate tetrahedra. X-ray diffraction (XRD) studies indicate an orthorhombic crystal system, with structural similarities to ammonium phosphate due to the analogous tetrahedral geometry of AsO43- and PO43-. Acid salts, such as diammonium arsenate ((NH4)2HAsO4) and ammonium dihydrogen arsenate (NH4H2AsO4), form under specific conditions and exhibit greater acidity. Aqueous solutions of ammonium arsenate are mildly acidic (pH ~4–6) and can neutralize bases with moderate exothermic release. The compound is highly soluble in water but decomposes in hot water, yielding arsenious oxides and ammonia gas.[3][2][5]
Synthesis
Ammonium arsenate is synthesized by reacting concentrated arsenic acid (H3AsO4) with ammonia (NH3) in aqueous solution, resulting in the precipitation of colorless trihydrate crystals.
The reaction proceeds at room temperature to prevent decomposition:
- H3AsO4 + 3NH3 → (NH4)3AsO4 + 3H2O
Acid salts, such as diammonium arsenate, are produced by adjusting the ammonia-to-arsenic acid ratio (e.g., 2:1). Purification involves recrystallization, with composition verified by inductively coupled plasma mass spectrometry (ICP-MS) or Fourier-transform infrared spectroscopy (FTIR), which detect As–O and N–H vibrational modes. Industrial synthesis is uncommon due to toxicity, but laboratory preparation supports studies in arsenic speciation and toxicology. Heating above 100 °C releases ammonia, forming ammonium hydrogen arsenate or arsenic oxides, necessitating precautions to avoid toxic fumes.[2][5][6]
Analysis
Quantifying ammonium arsenate in environmental and biological samples demands precise techniques due to its low concentrations and matrix interferences. Ion exchange chromatography coupled with inductively coupled plasma mass spectrometry (LC-ICP-MS) is the gold standard for speciating arsenate and arsenite, achieving detection limits in the parts-per-billion range. X-ray absorption spectroscopy (XAS) determines arsenic oxidation states (As(V) vs. As(III)) in solids or solutions. Fourier-transform infrared spectroscopy (FTIR) detects As–O and N–H bonds, confirming the compound's presence. Environmental samples are extracted using hydroxylammonium hydrochloride or ammonium oxalate to preserve speciation, followed by high-performance liquid chromatography (HPLC) with hydride generation-atomic fluorescence spectroscopy (HG-AFS). Radioisotope assays with [73As]arsenate track microbial transformations in soils, aiding biogeochemical studies. These methods are essential for monitoring contamination and assessing exposure risks.[5]
Recent studies have developed portable whole-cell biosensors (WCBs) with ArsR-regulated luciferase genes, detecting arsenate in soils at 10–50 μg/L.[7]
Geologic rarity
Ammonium arsenate is extremely rare in nature, as ammonium and arsenate ions are typically found in distinct geochemical settings. Trace occurrences have been documented in arsenic-rich environments, such as acid mine drainage (AMD) sites or geothermal springs, where microbial activity or industrial runoff introduces ammonia and arsenate. For instance, in AMD from gold mines in Dushan, Guizhou, China, transient ammonium arsenate-like phases form in sulfate-rich waters with elevated ammonium from fertilizer runoff. These are minor compared to arsenate minerals like scorodite (FeAsO4·2H2O) or tooeleite (Fe6(AsO3)4SO4(OH)4·4H2O). The compound's instability under typical environmental pH and redox conditions favors arsenite or sulfide phases, limiting its natural prevalence.[7]
Nevertheless, ammonium arsenate may form biogenically. A 2023 study on rice paddies in Guizhou, China, identified anaerobic bacterial metabolism that coupled ammonium oxidation to arsenate reduction (As-ammox).[7]
Remove ads
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads